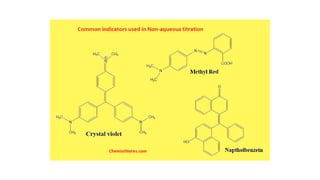
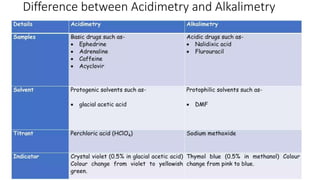
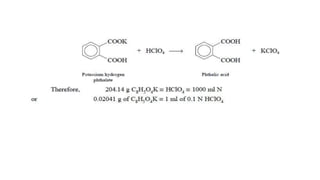
Non-aqueous titration is a volumetric analysis performed in solvents without water, allowing for accurate determination of endpoints due to the absence of water interference. It is particularly useful for titrating very weak acids and bases and employs various types of solvents including aprotic, protophilic, protogenic, and amphiprotic. The method has advantages like producing precise results and being applicable in pharmaceutical assays, though it also has drawbacks such as lower solvent stability compared to aqueous options.