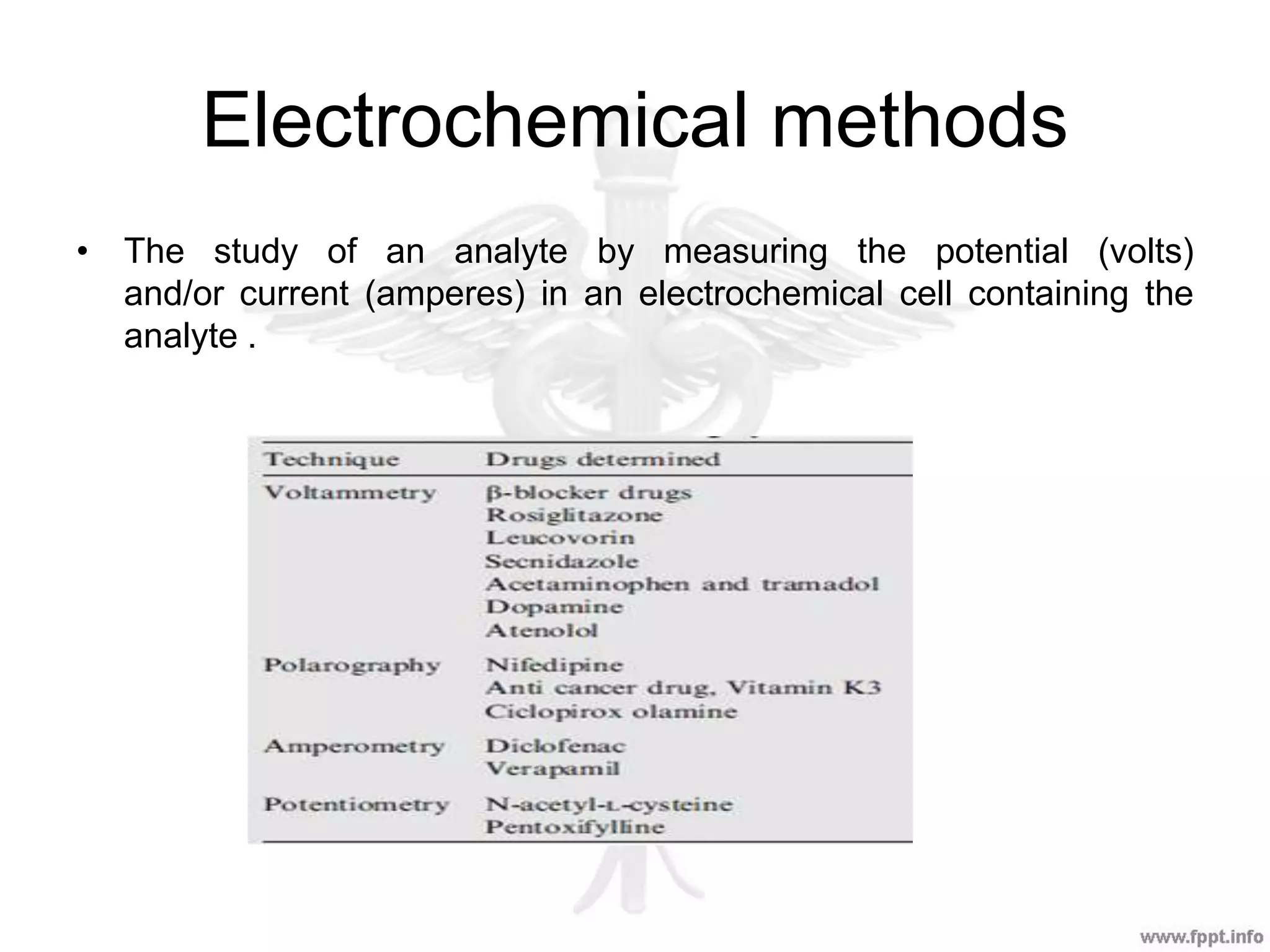
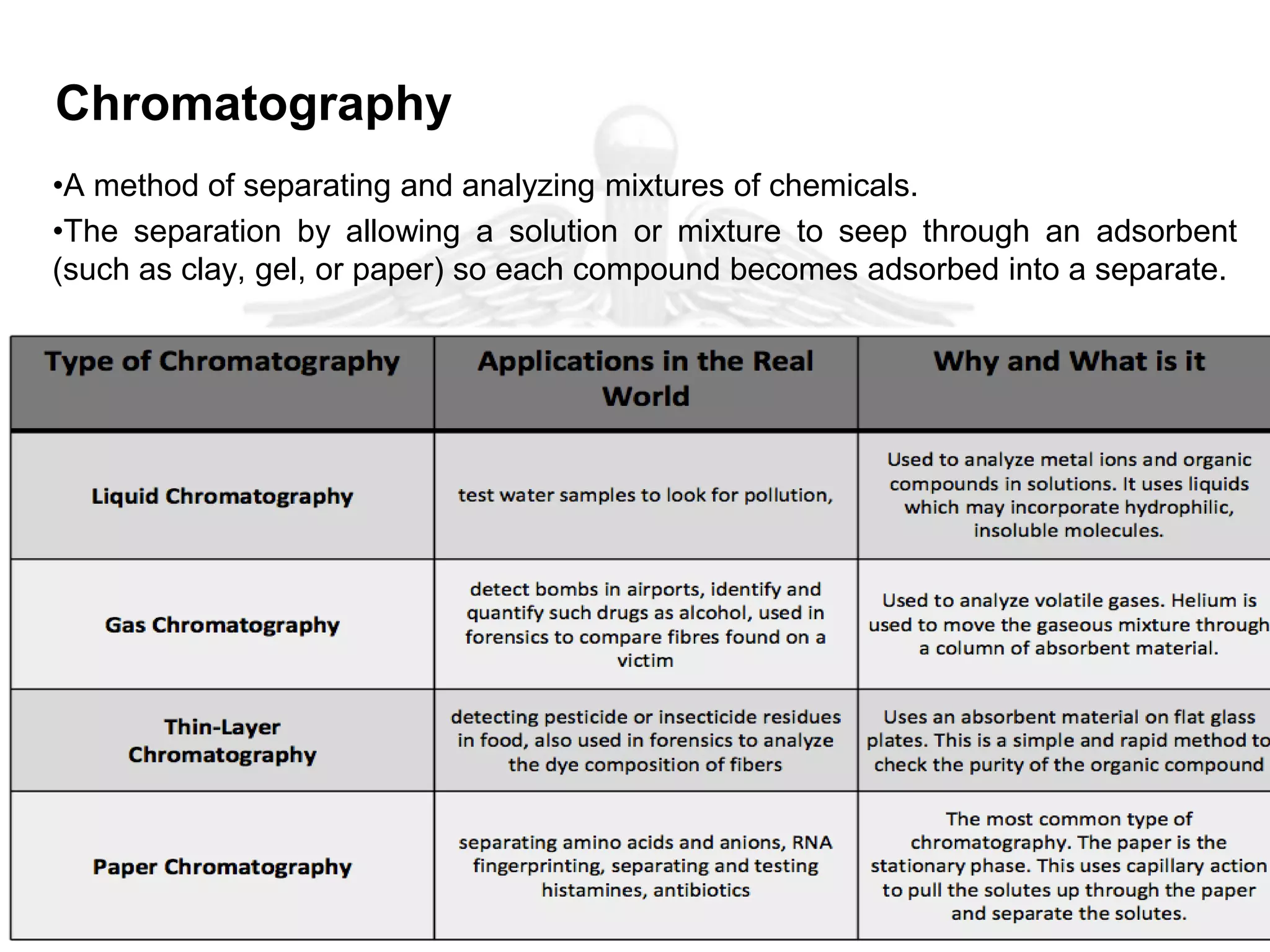
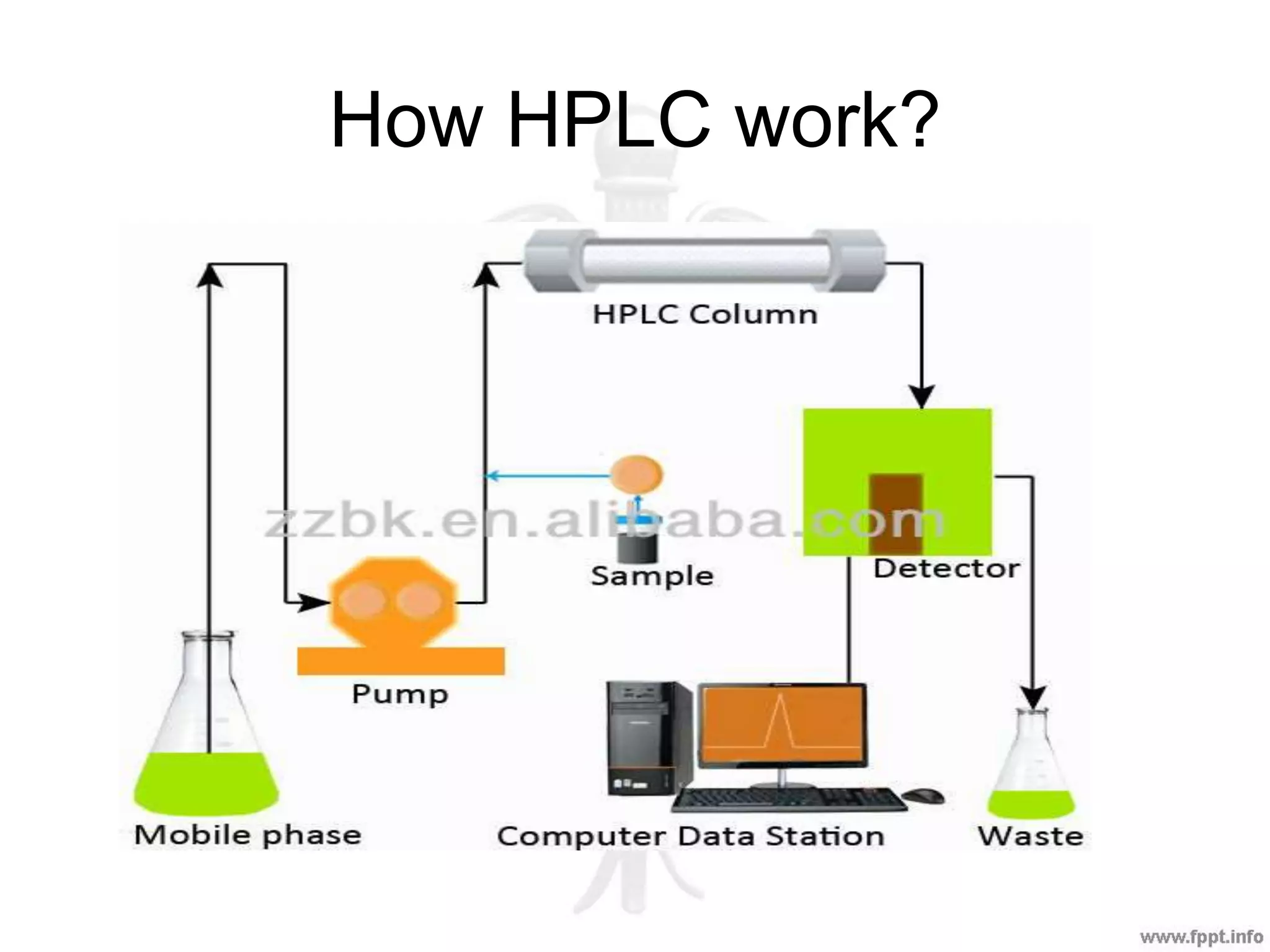
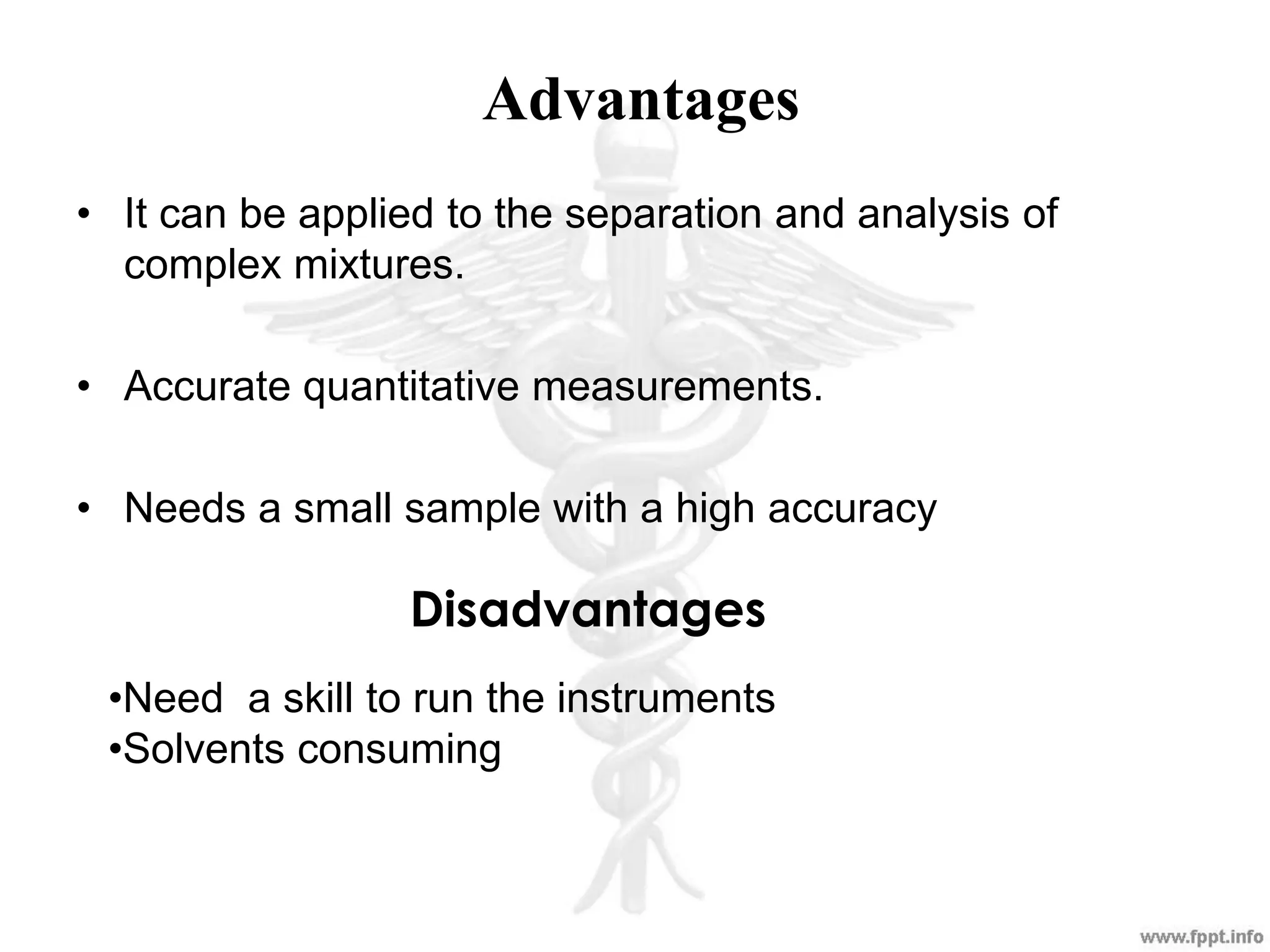
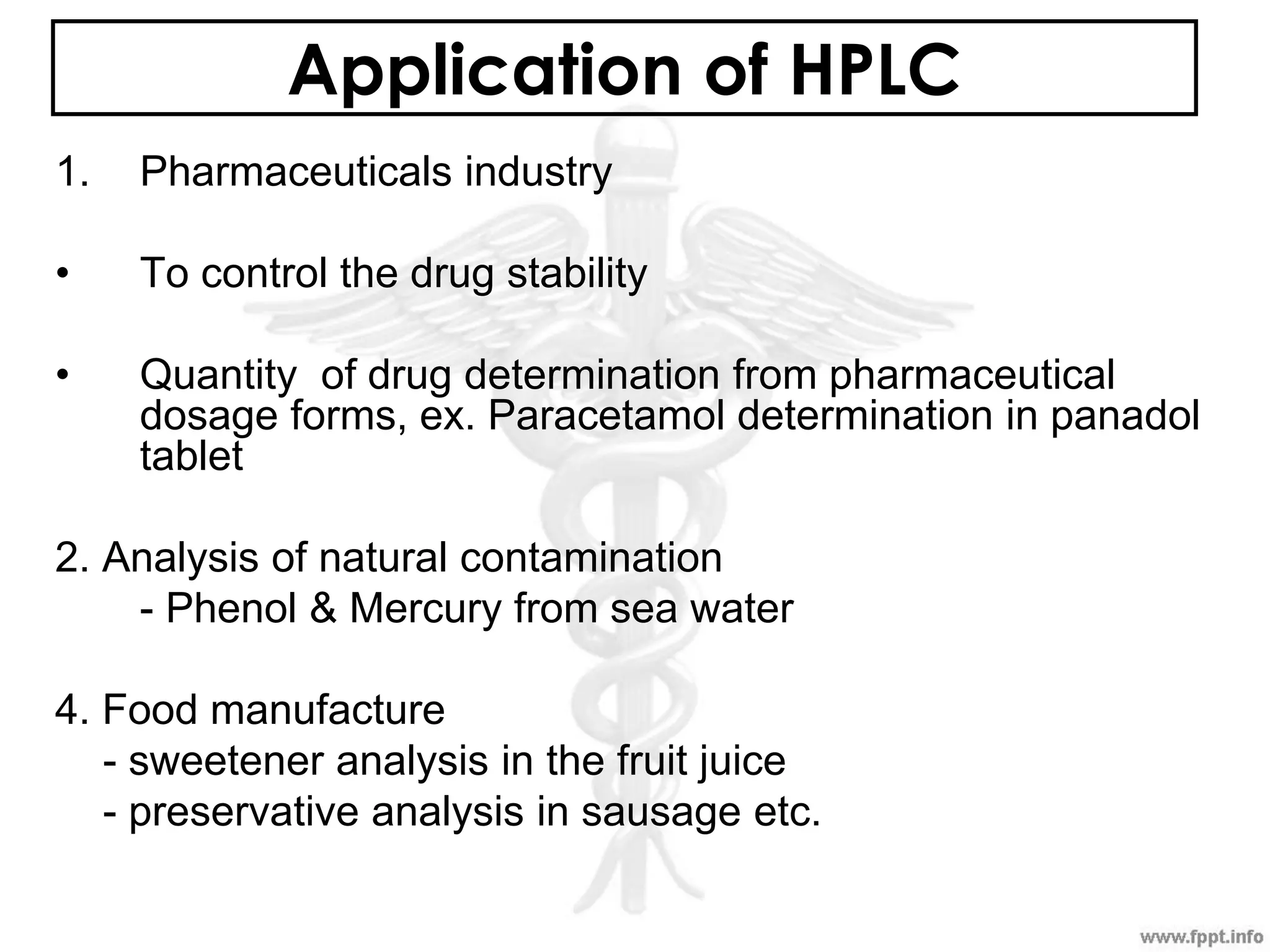
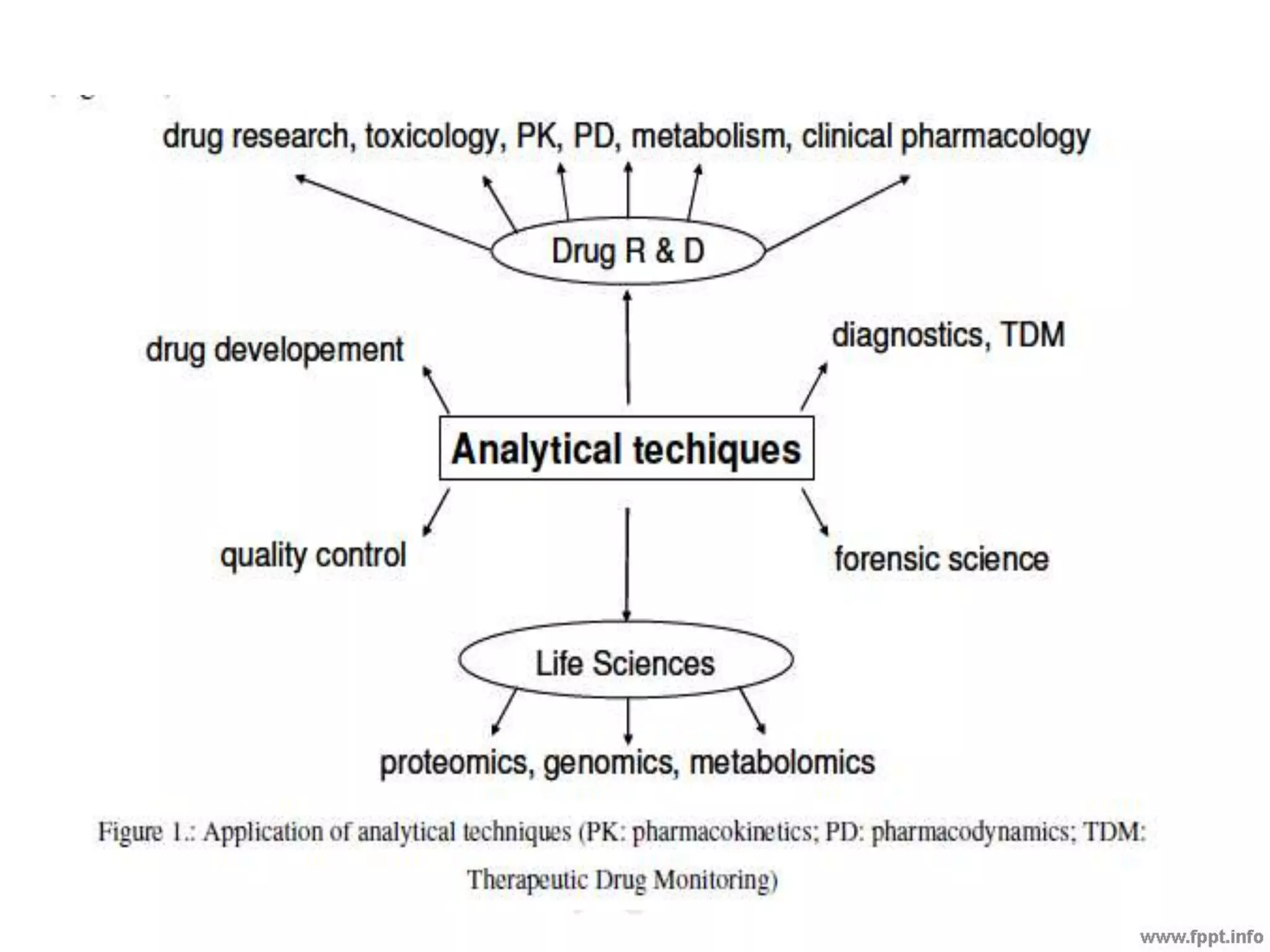
The document outlines various analytical techniques used in pharmaceutical analysis, including titrimetric, chromatographic, spectroscopic, electrochemical, and electrophoretic methods. It highlights specific methods such as high-performance liquid chromatography (HPLC) and spectroscopy for their efficiency in analyzing mixtures and determining the quality and composition of substances. Applications of these techniques span the pharmaceutical industry, food manufacturing, and environmental analysis.