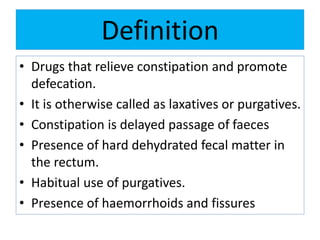
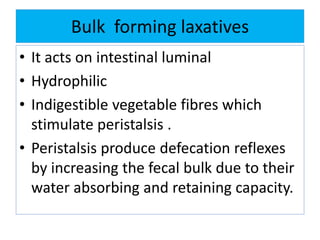
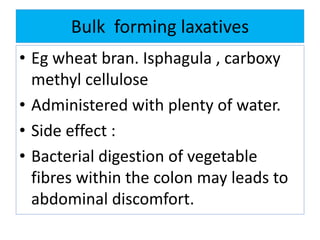
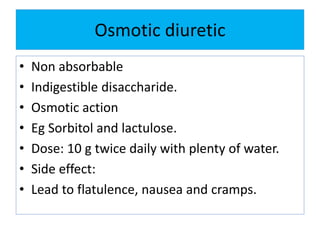
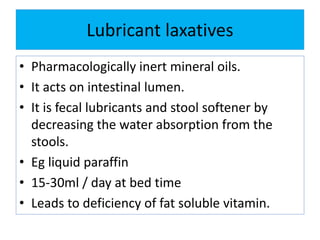
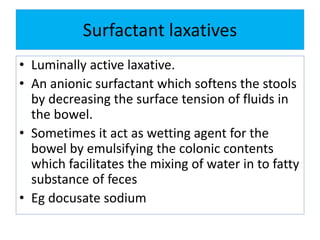
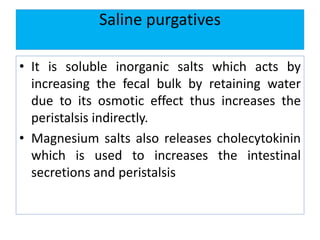
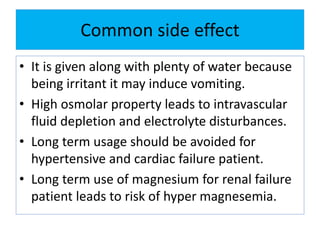
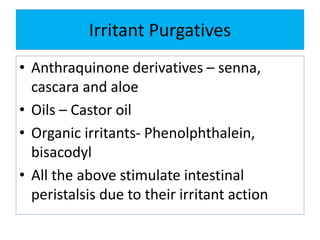
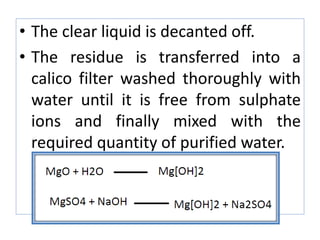
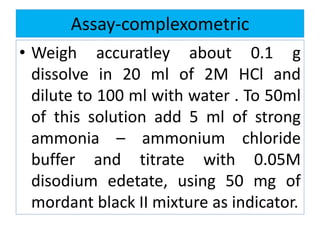
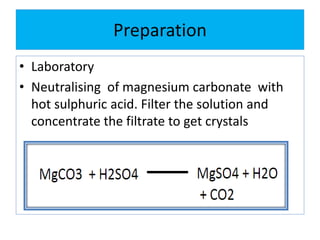
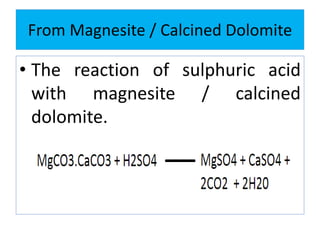
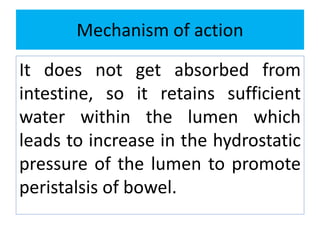
This document discusses various types of saline cathartics (laxatives) including their definitions, mechanisms of action, preparations, and uses. The main types discussed are bulk forming laxatives, osmotic laxatives, lubricant laxatives, surfactant laxatives, and purgatives. Specific saline cathartics described in detail include magnesium hydroxide, magnesium sulfate (Epsom salt), and magnesium carbonate. Their preparations, assays, uses as laxatives or other purposes, and dosages are provided.




















![uses
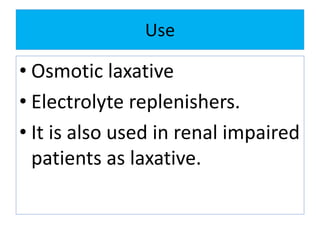
• Antacid
• Osmotic laxative
• Pharmaceutical aid
• Slow acting antacid. [ during
pregnancy and lactation]
• Cathartic](https://image.slidesharecdn.com/cathartics-180217081223/85/Cathartics-38-320.jpg)

