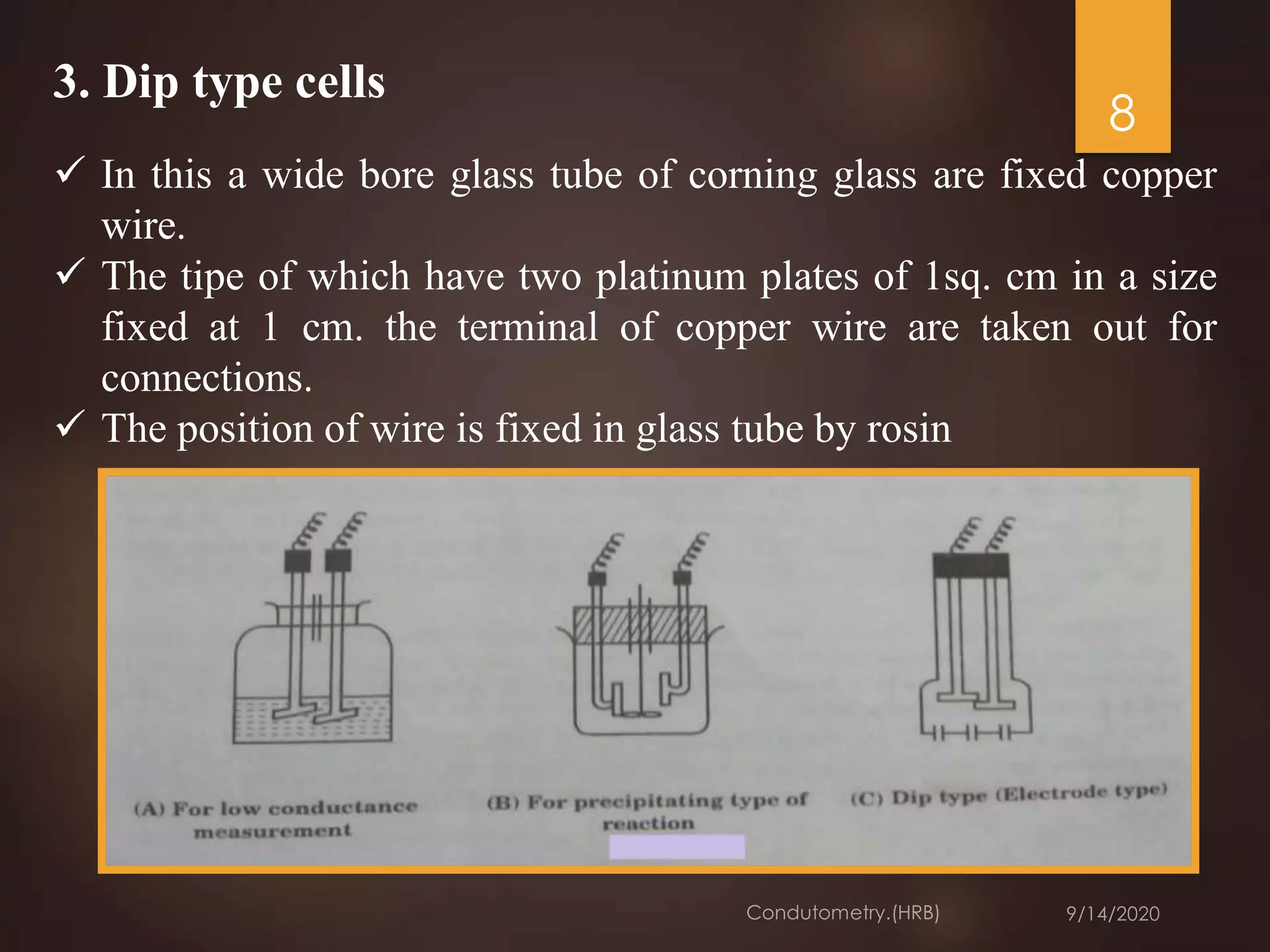
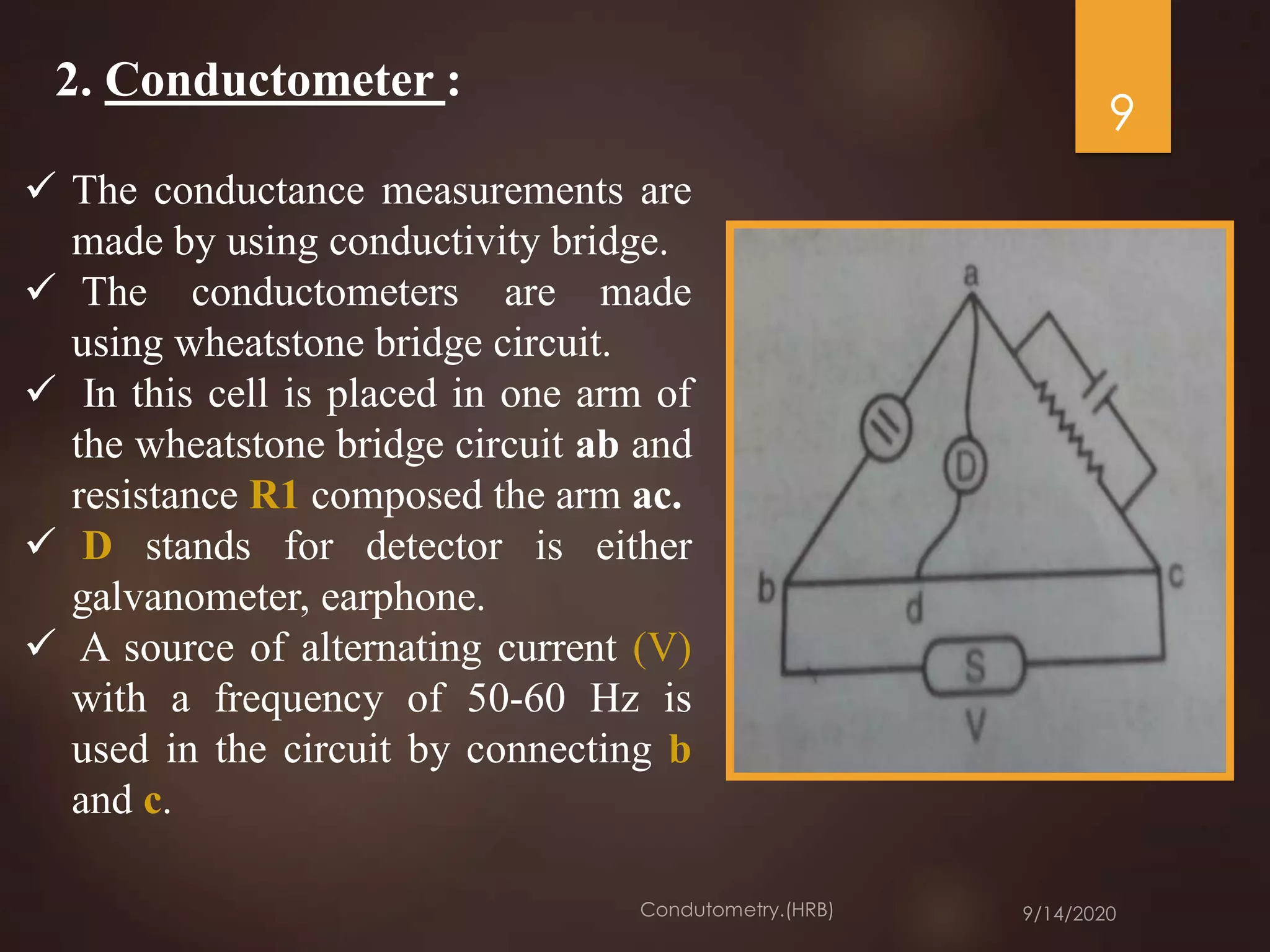
This document provides an overview of conductometry. It discusses how conductometry measures the conductance of electrolyte solutions using a conductivity cell and conductometer. It describes different types of conductivity cells and how conductometric titrations work by measuring changes in conductance during titrations. Examples of various acid-base titrations are given. Conductometric titrations can be used to analyze many different samples and have advantages like not requiring indicators. Applications include measuring water pollution, food analyses, and more.