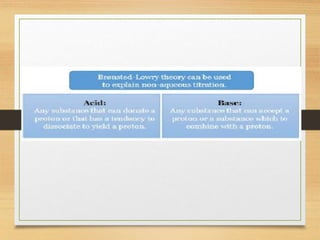
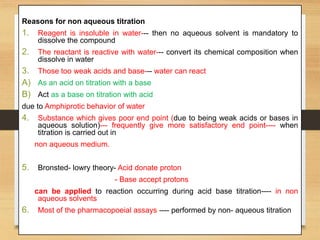
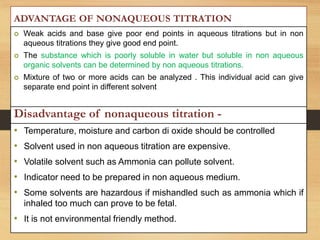
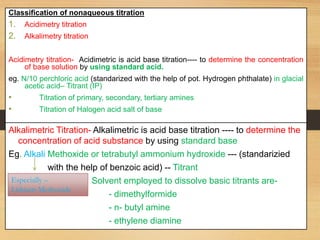
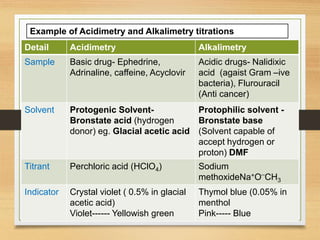
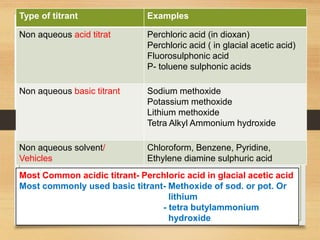
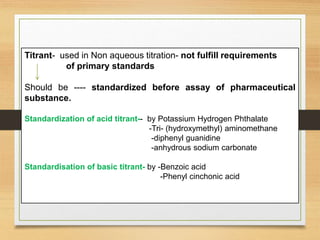
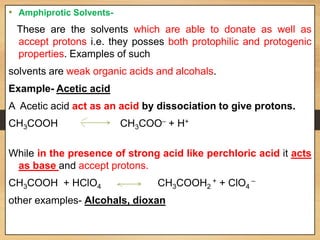
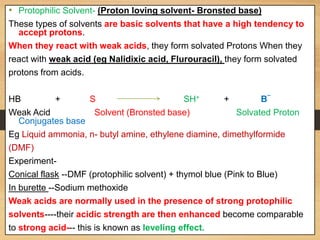
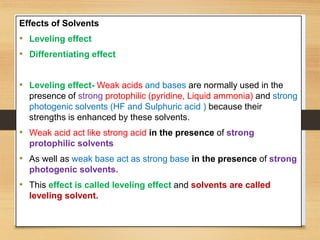
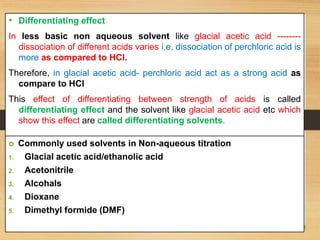
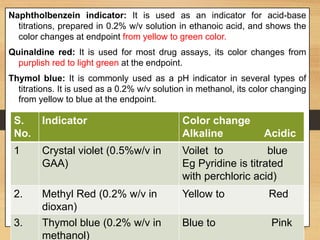
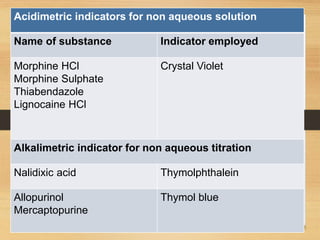
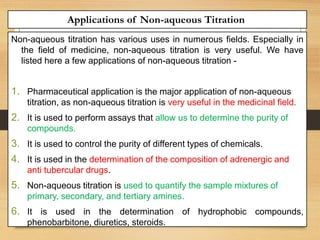
The document provides a comprehensive overview of non-aqueous titration, detailing its principles, types, solvents, and methods for determining endpoints. It highlights the advantages of using non-aqueous solvents for titrating weak acids and bases, while addressing the associated disadvantages and potential hazards. Applications of non-aqueous titration mainly focus on pharmaceutical assays and determining the purity and composition of various compounds.