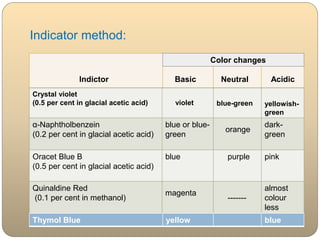
This document discusses non-aqueous titration, which involves titrating weakly acidic or basic substances using non-aqueous solvents to obtain a sharp endpoint. It describes the different types of non-aqueous solvents that can be used, including aprotic, protophilic, protogenic, and amphiprotic solvents. The document also discusses how solvent properties affect acidity and outlines methods for titrating weak acids and bases via potentiometric or indicator methods. Key indicators and solvents used for titrating each are provided.