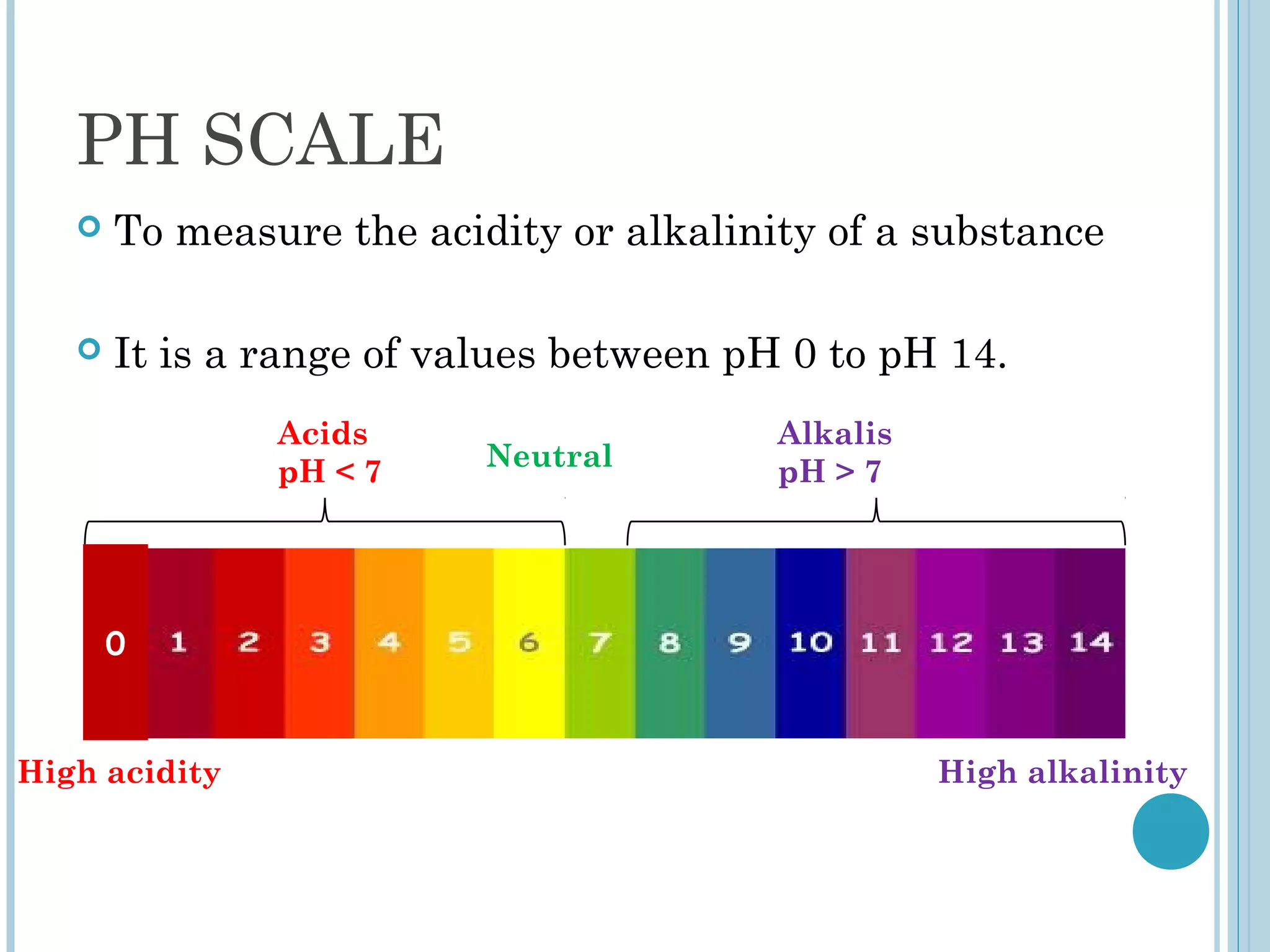
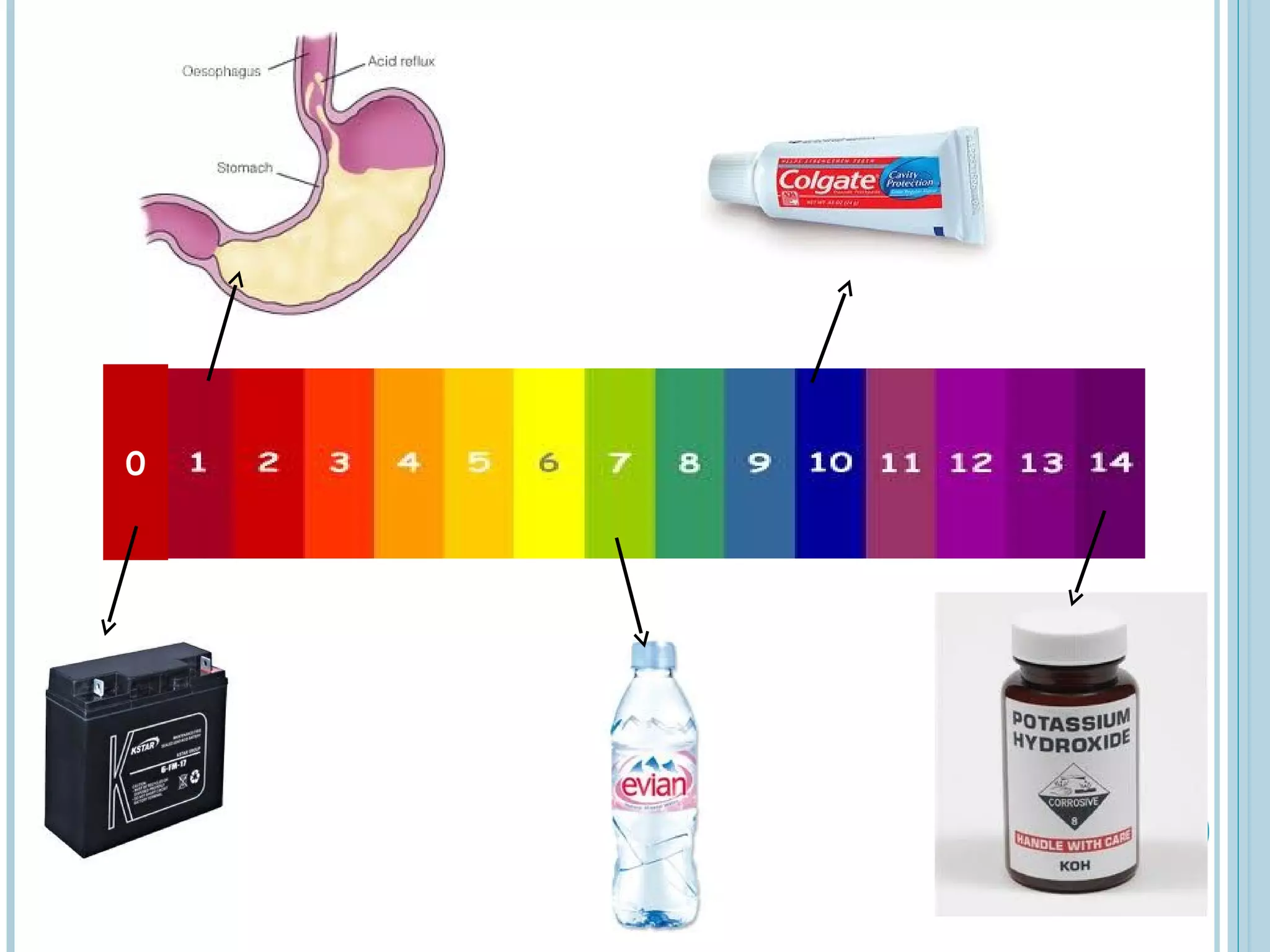
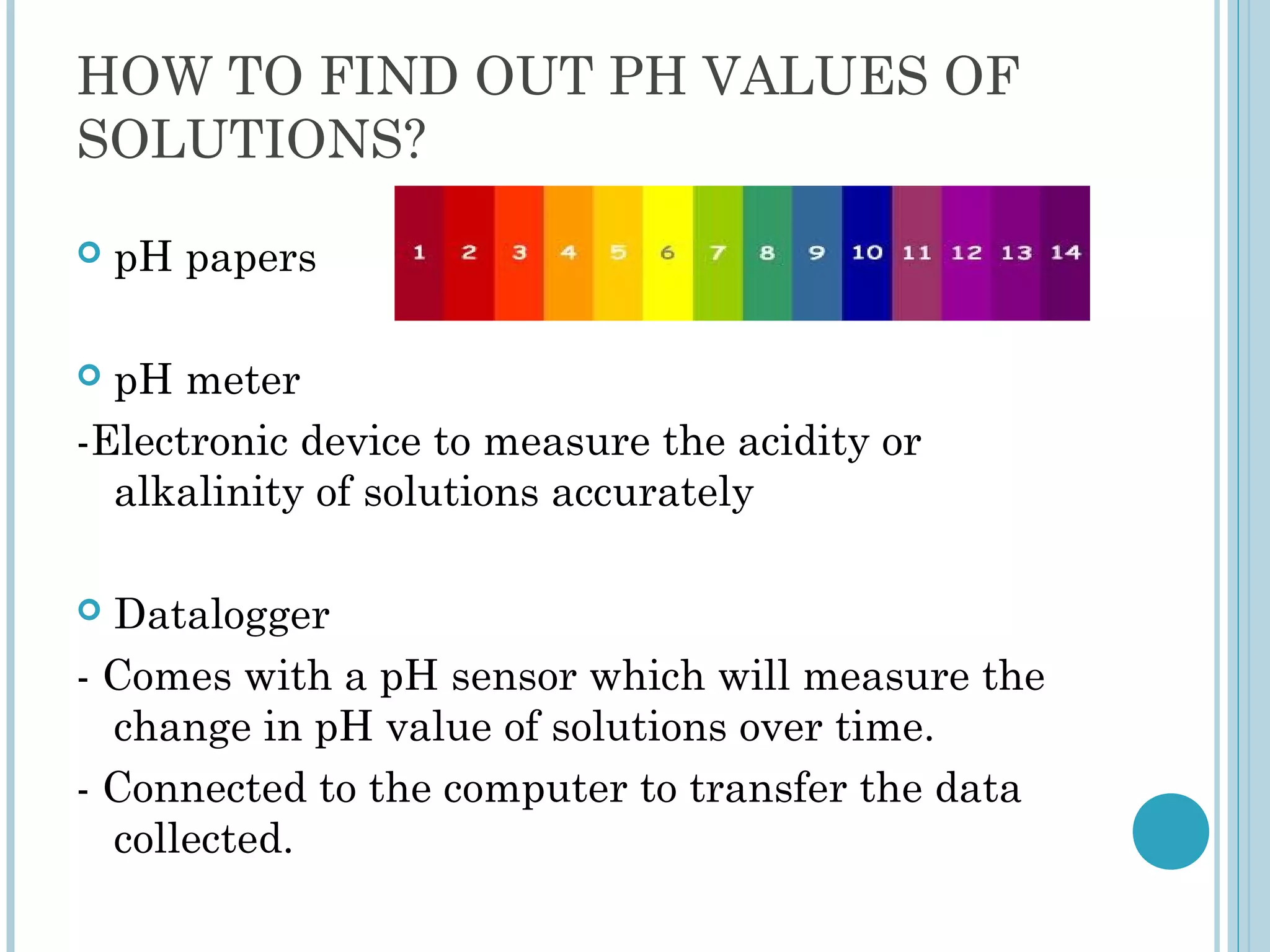
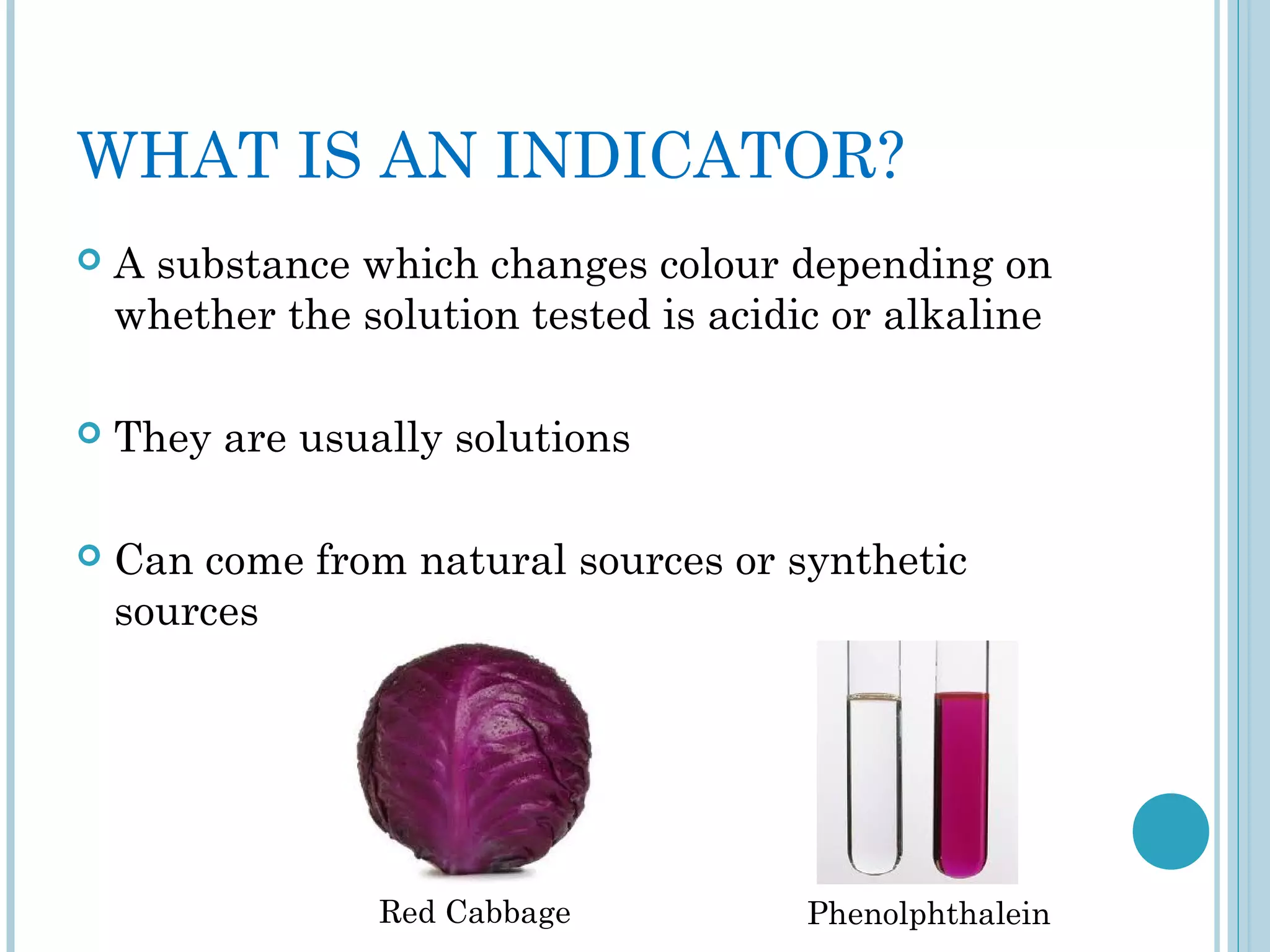
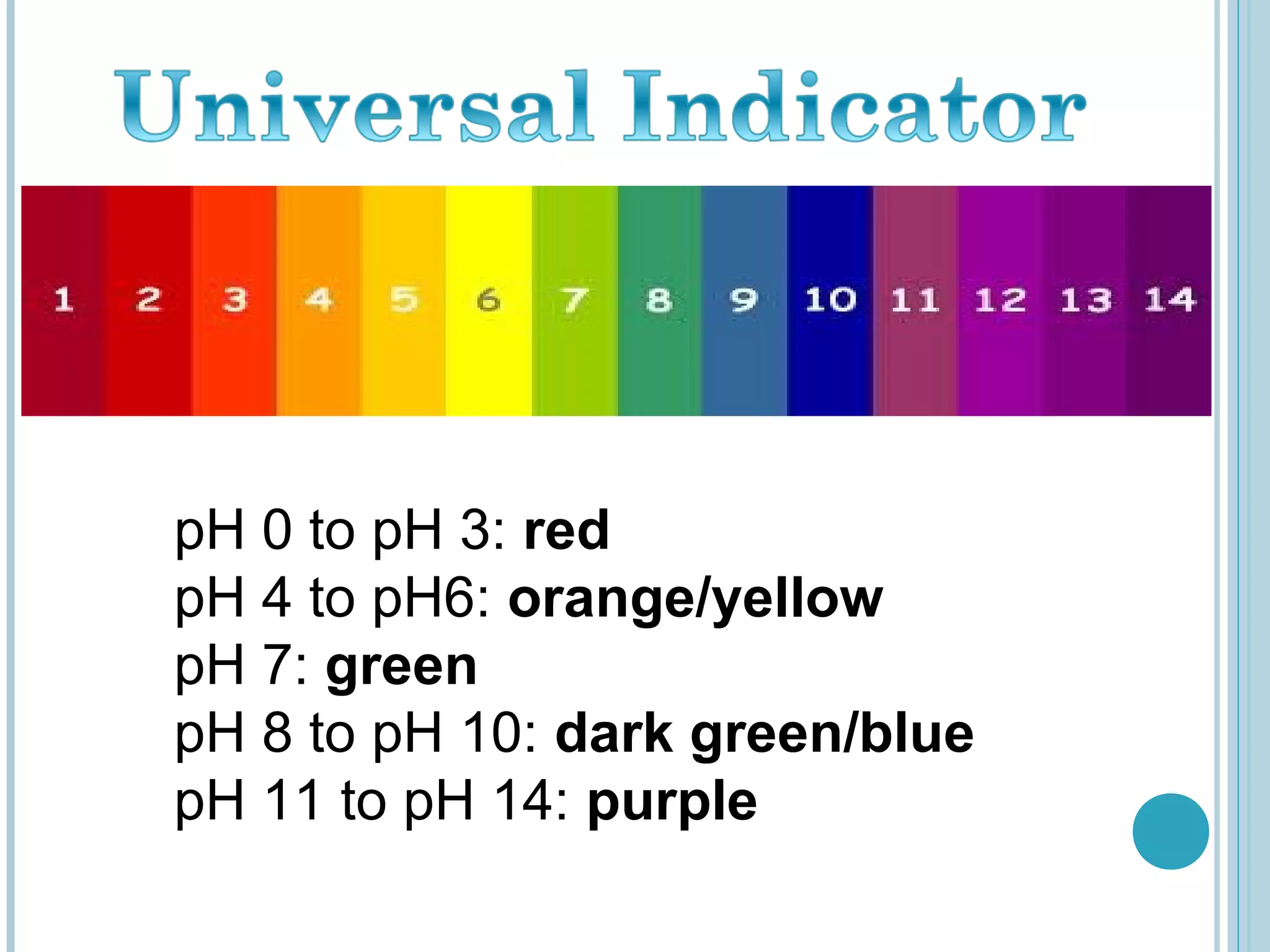
This document discusses pH and indicators. It explains that pH is a measure of acidity or alkalinity on a scale from 0 to 14, with acids below 7 and alkalis above. It describes common tools to measure pH like pH papers, meters, and dataloggers. It also defines indicators as substances that change color based on pH and lists common natural and synthetic indicators like litmus, universal indicator, methyl orange, and phenolphthalein.