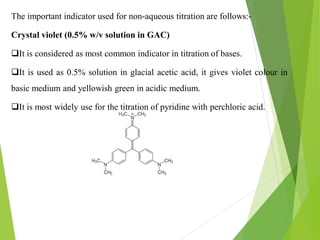
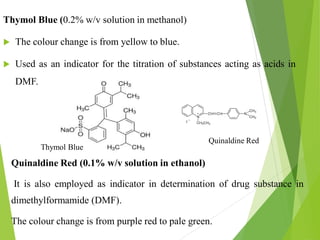
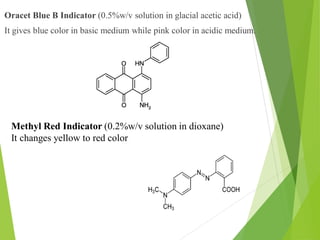
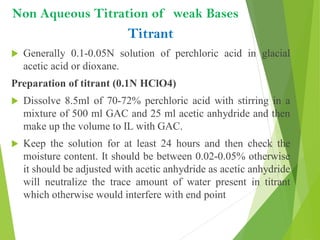
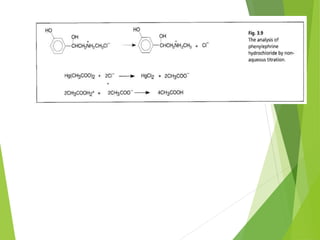
Non-aqueous titrations involve using a non-aqueous solvent instead of water. They are used when the reactant is insoluble in water, reactive with water, or too weak an acid or base. The document discusses different types of non-aqueous solvents and their properties, as well as considerations for non-aqueous titration procedures and methods. Common solvents, titrants, and indicators used are outlined. Examples of titrating weak bases with perchloric acid in acetic acid and weak acids with sodium methoxide are provided.