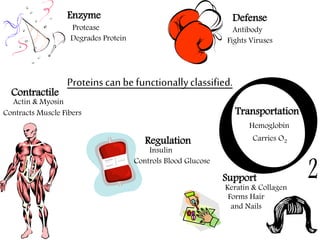
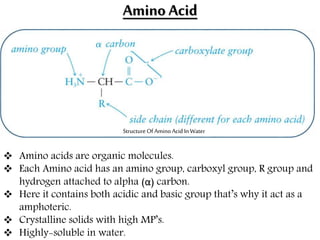
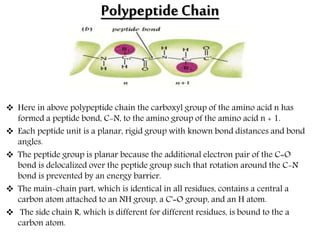
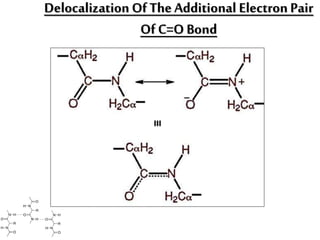
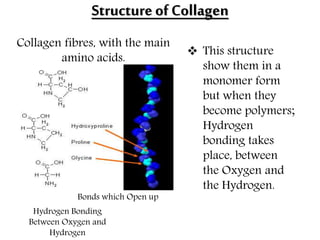
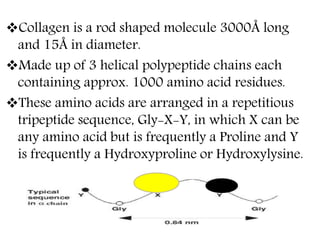
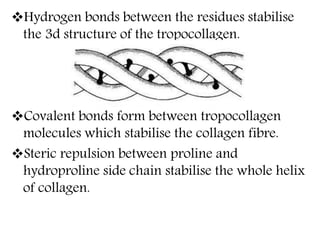
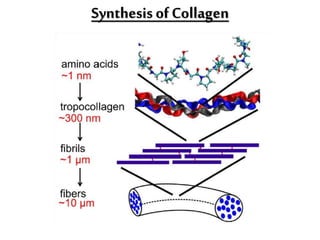
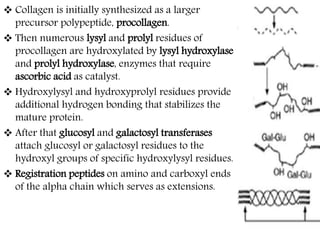
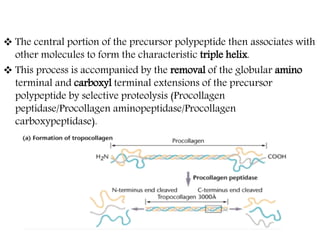
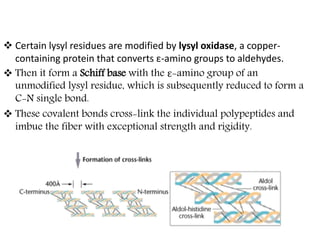
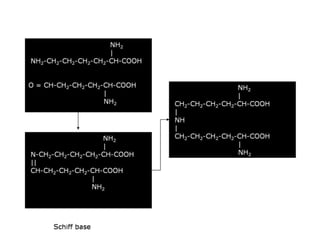
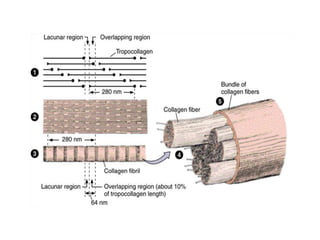
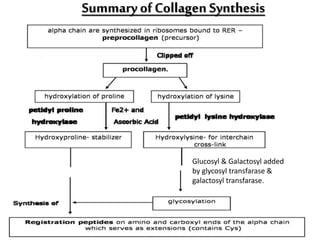
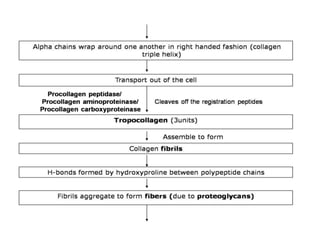
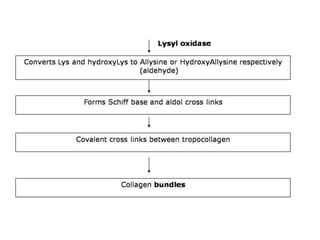
This document discusses different types of fibrous proteins. It begins by introducing proteins and describing their structure as polypeptide chains formed from amino acids linked by peptide bonds. It then focuses on three important fibrous proteins: collagen, elastin, and keratin. For each, it describes their structure, synthesis, properties, and applications. Collagen is the most abundant protein and forms connective tissues. Elastin provides elasticity. Keratin forms hair, nails, feathers and horns through intermolecular bonding. These proteins each have distinct structures and functions that make them vital components of living tissues.