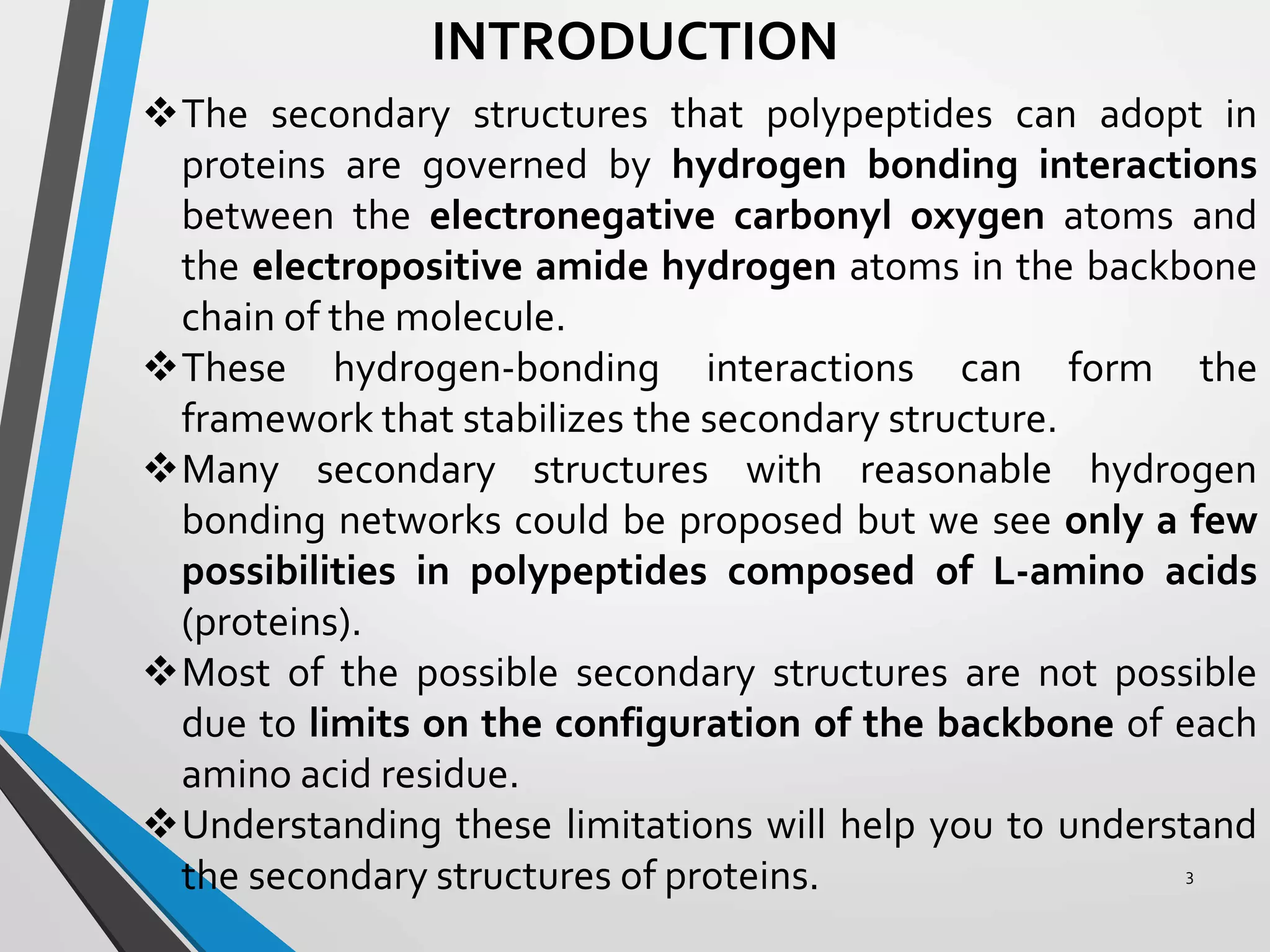
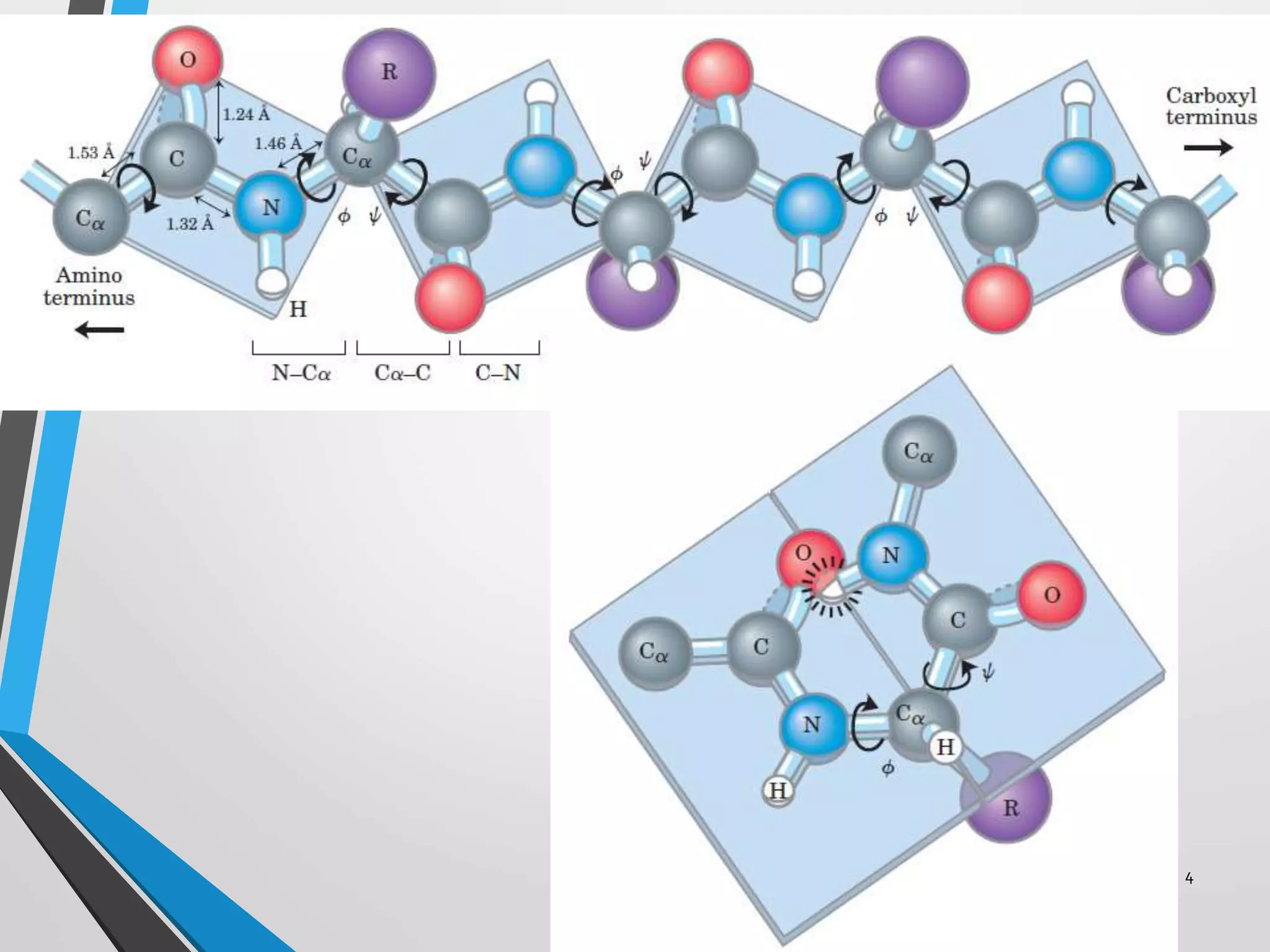
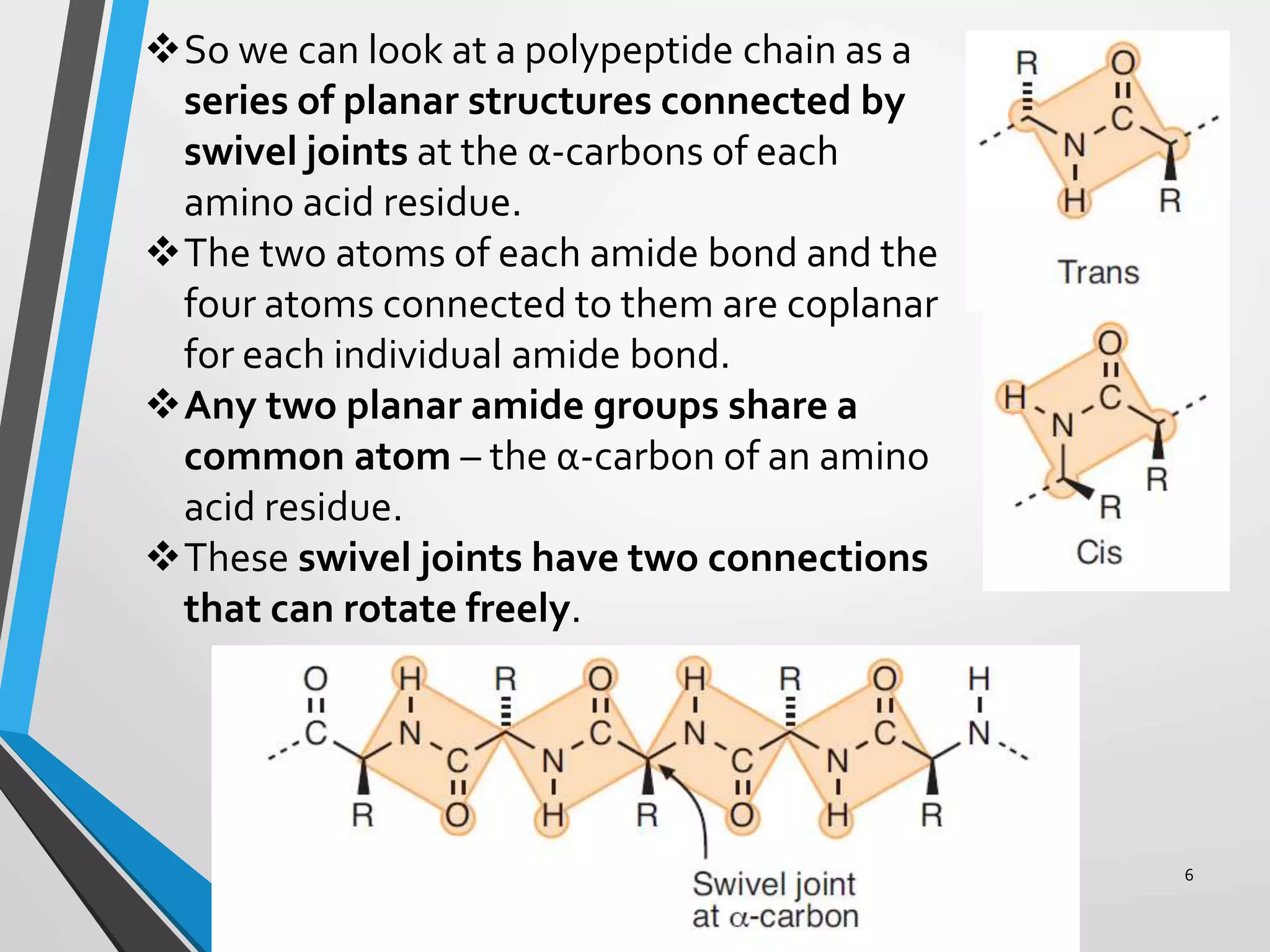
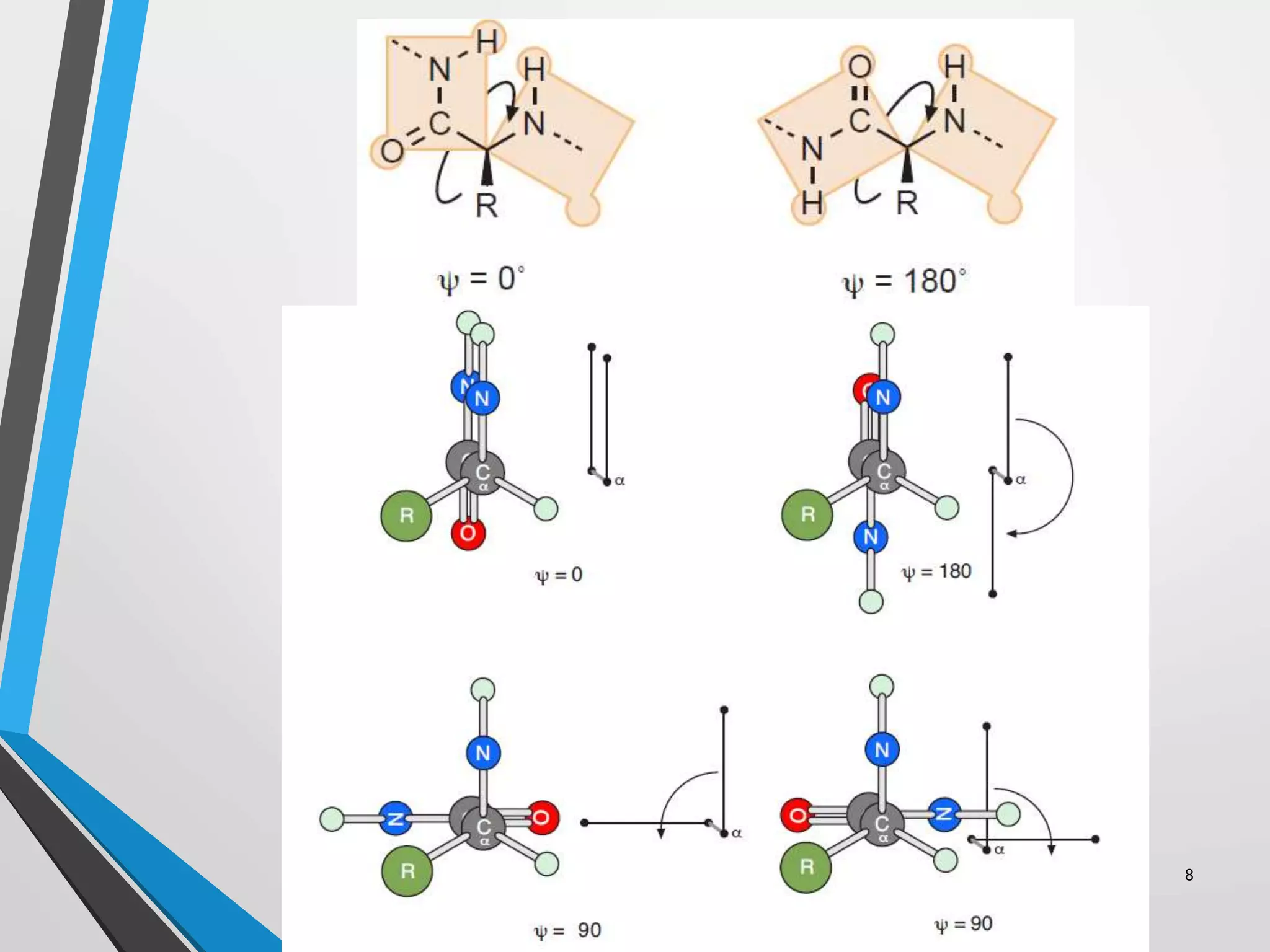
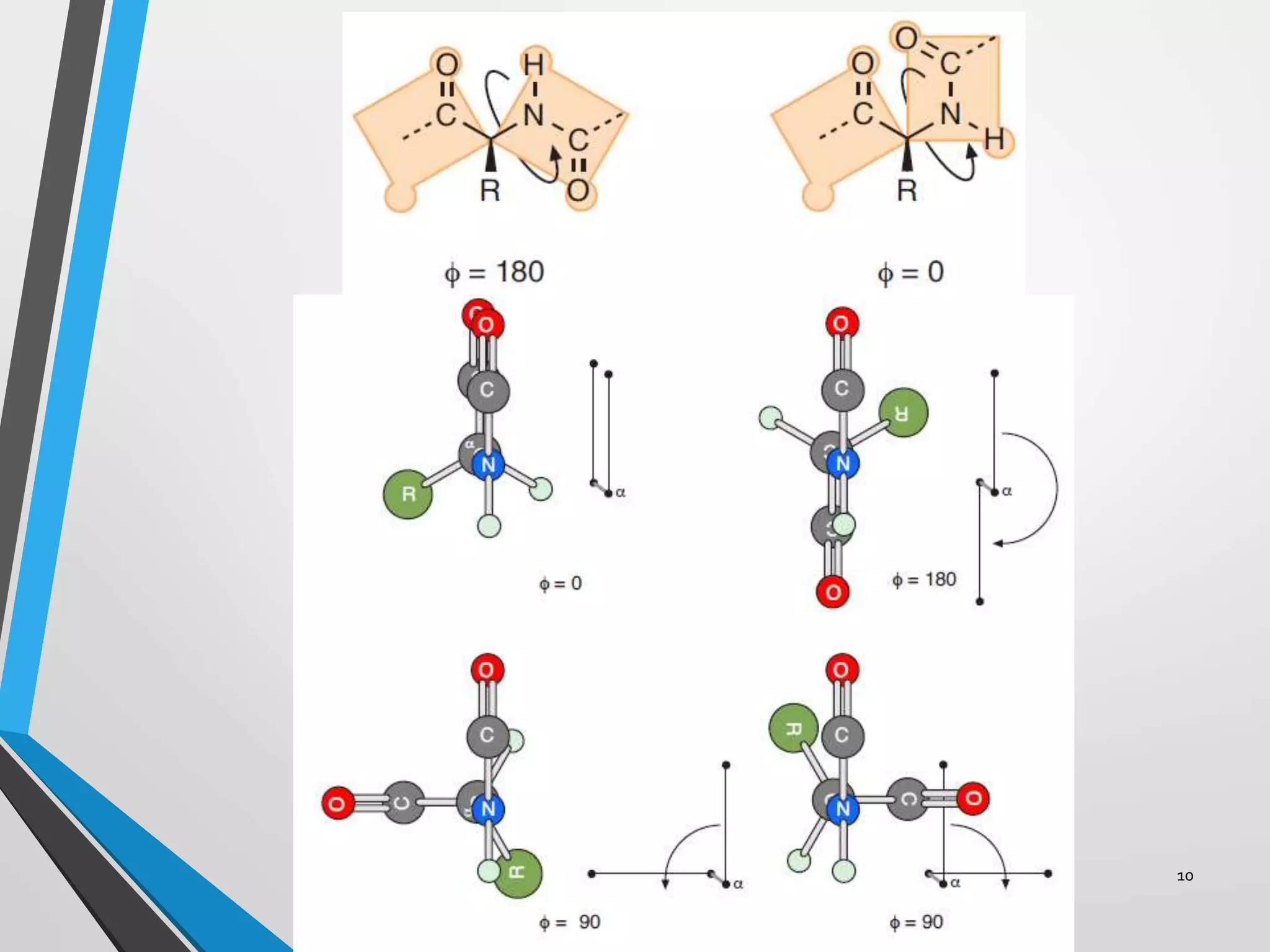
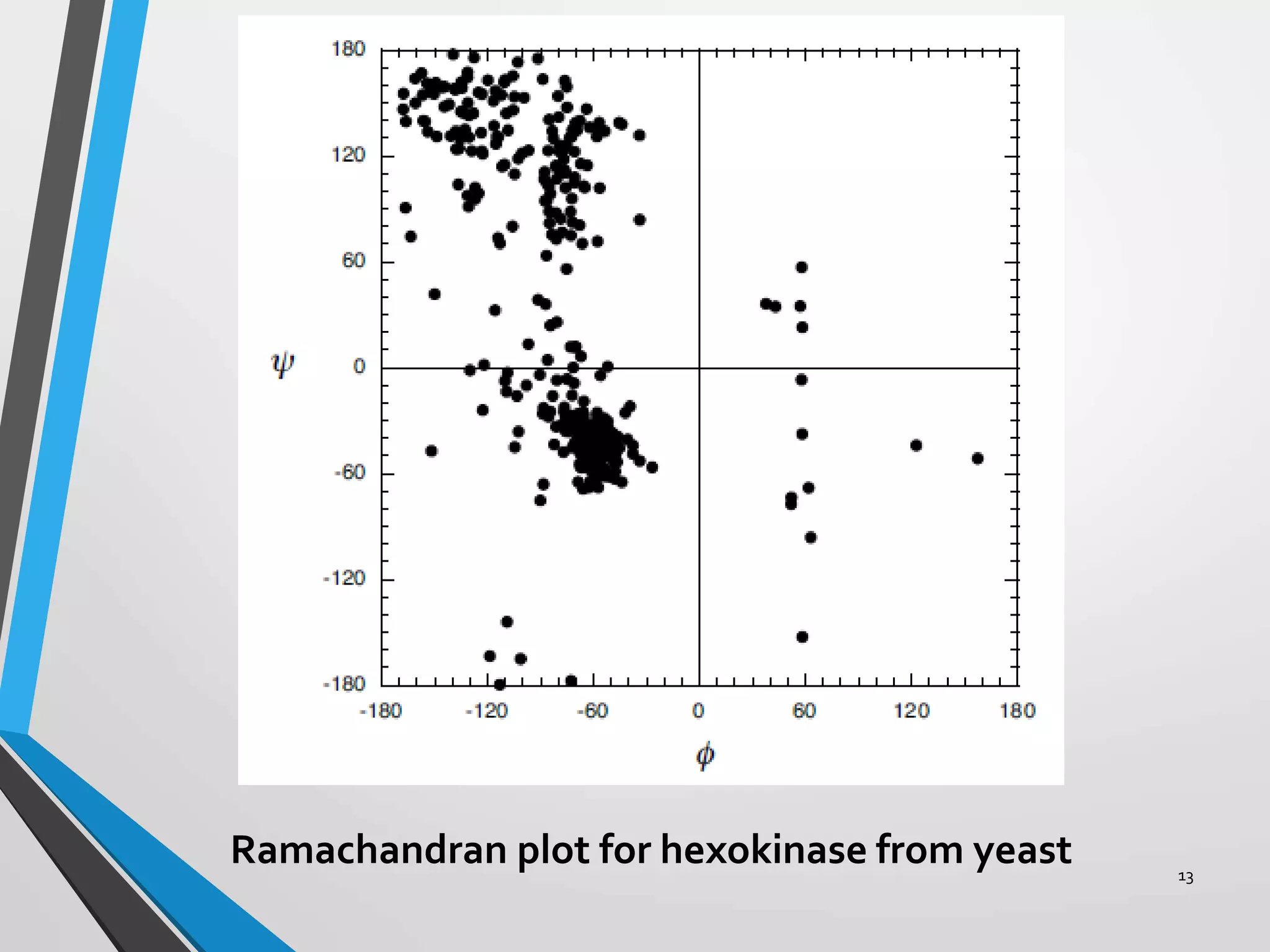
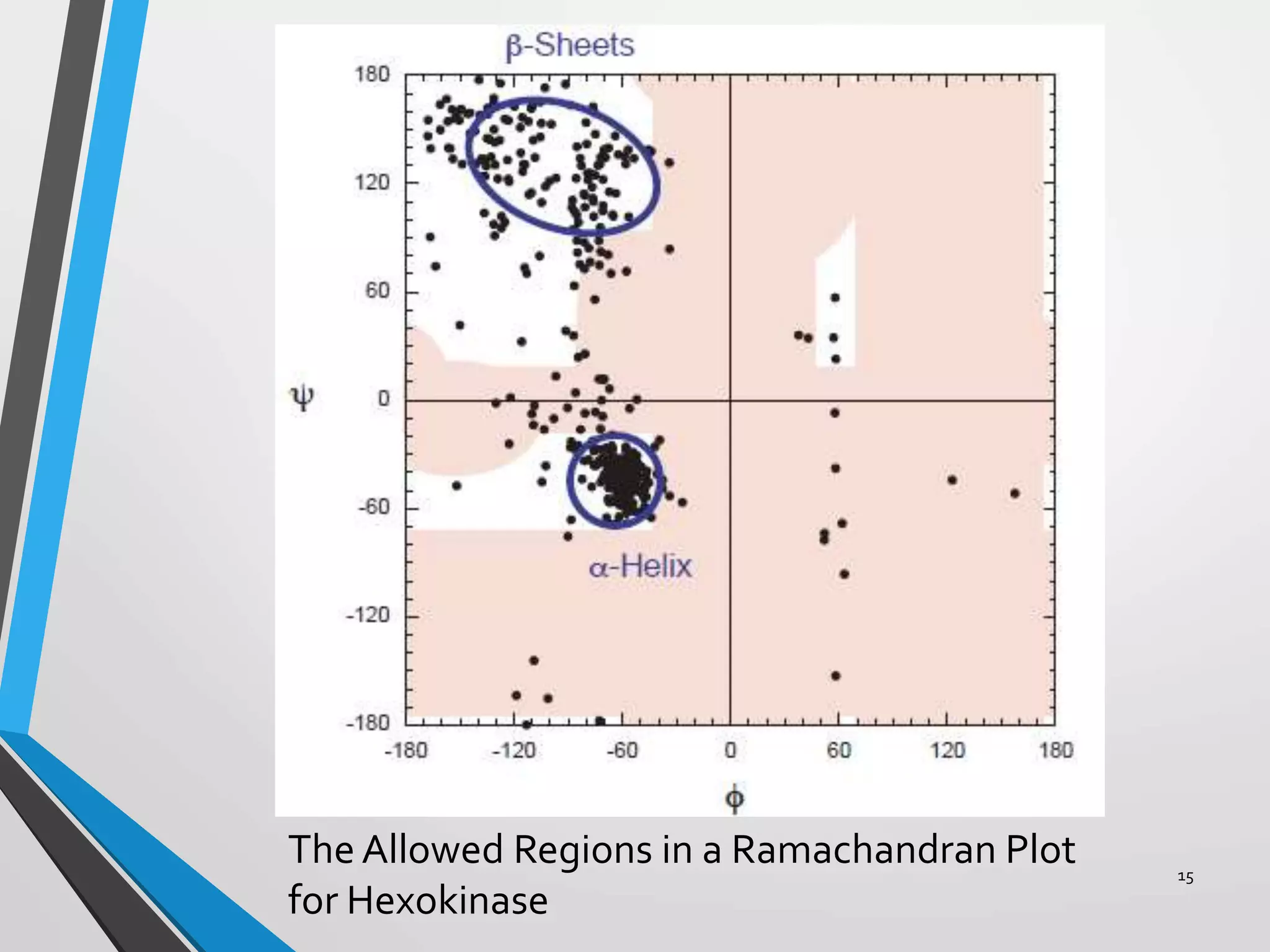
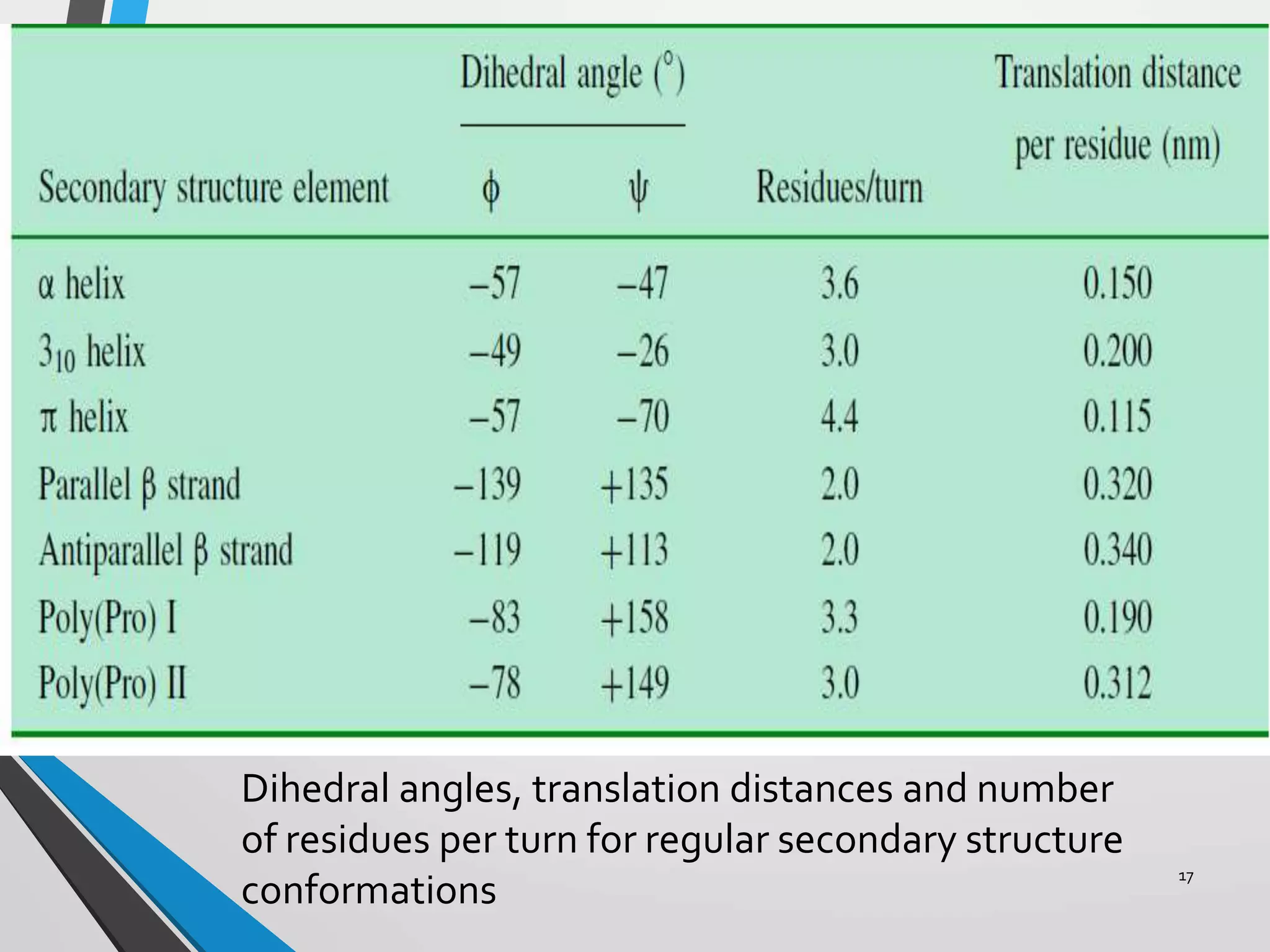
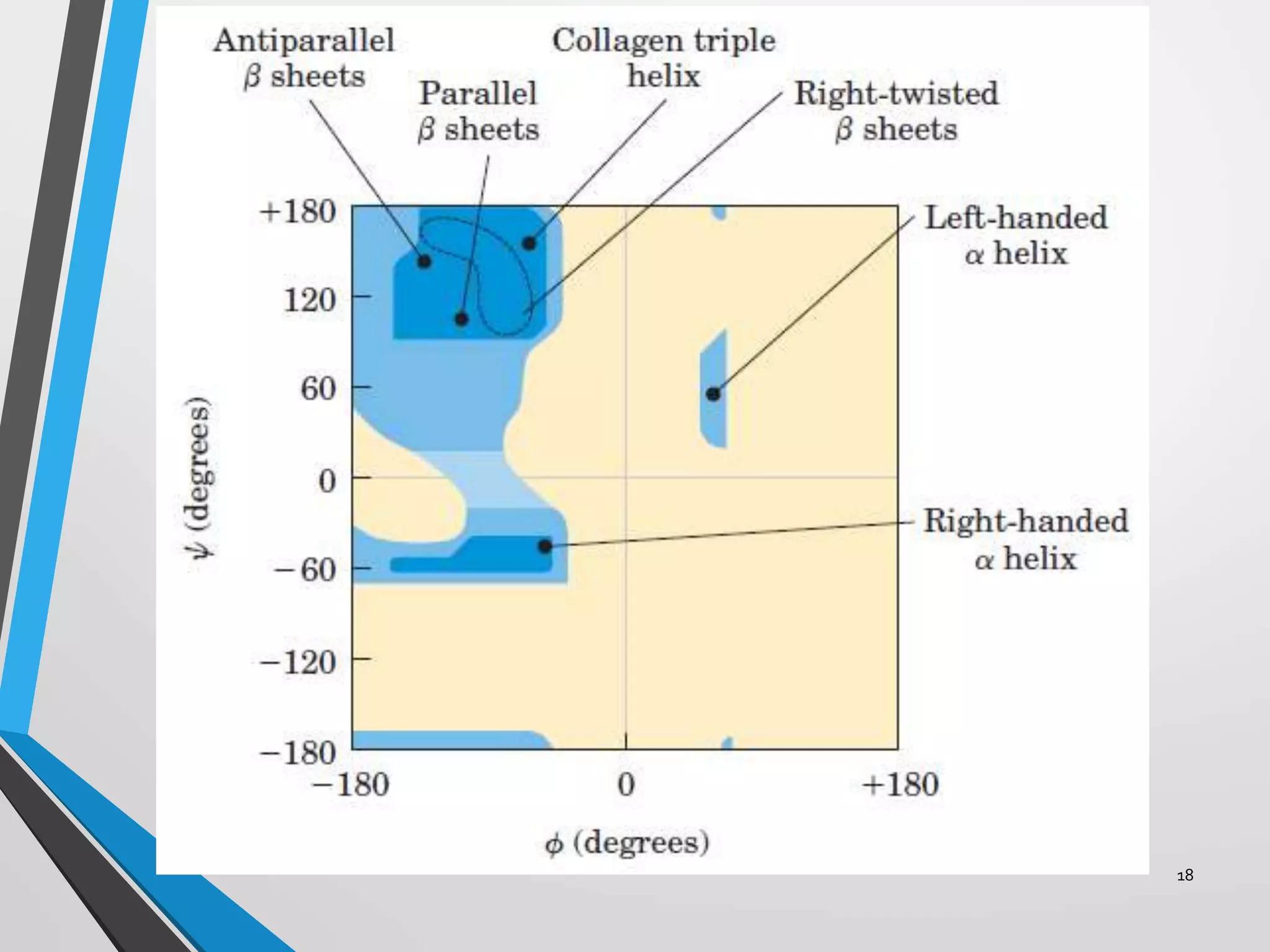
This document discusses the limits on rotation in protein backbones and defines the psi (ψ) and phi (φ) angles. It introduces the Ramachandran plot, which maps allowed combinations of ψ and φ angles based on steric constraints. The plot reveals preferred regions that correspond to common secondary structures like alpha helices and beta sheets. Understanding the steric limits on individual amino acid residues provides insight into how proteins fold into their specific three-dimensional shapes.