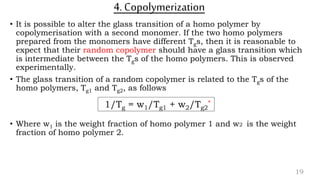
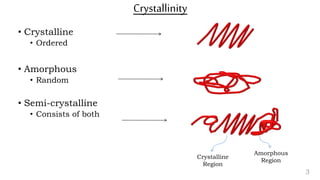
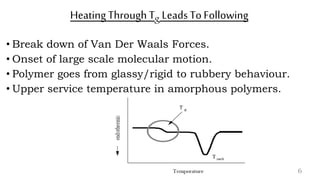
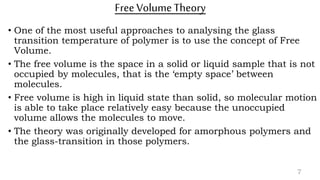
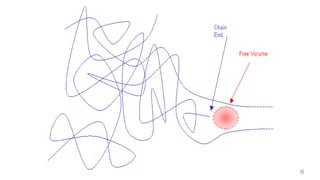
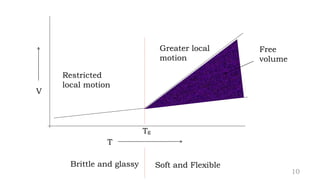
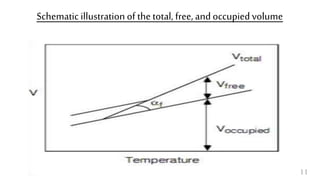
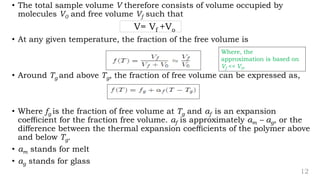
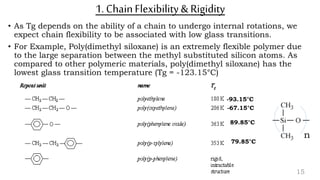
The document provides an overview of glass transition temperature (Tg) in polymers, discussing factors affecting it, including crystallinity, chain flexibility, and intermolecular forces. It explains the concepts of glassy and rubbery states, the free volume theory, and the distinction between crystalline, amorphous, and semi-crystalline polymers. Additionally, it outlines the impact of copolymerization and plasticizers on Tg, supported by relevant sources.







![2. StericEffects
• The presence of bulky side groups hinders rotation of the
backbone atoms due to steric hindrance, and therefore results in
an increase in Tg. The magnitude of this effect depends on the
size of the side groups.
• This is illustrated in the following Table for vinyl polymers
having the general structure,
—[CH2 — CHX ]—
17
-93.15°C
-20.15°C
99.85°C
134.85°C](https://image.slidesharecdn.com/glasstransitiontemperaturetg-dev-170305111643/85/Glass-transition-temperature-tg-18-320.jpg)
![3. Effect of Intermolecular Forces
• The presence of polar side groups leads to strong intermolecular
attractive interactions between chains which hinders molecular
motion thus causing an increase in Glass transition
temperature.
• This effect is illustrated in the following table for the polymers of
type −[CH2−CHX ]−
18
-20.15°C
80.85°C
84.85°C](https://image.slidesharecdn.com/glasstransitiontemperaturetg-dev-170305111643/85/Glass-transition-temperature-tg-19-320.jpg)