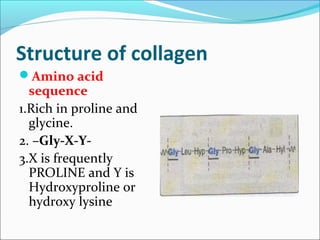
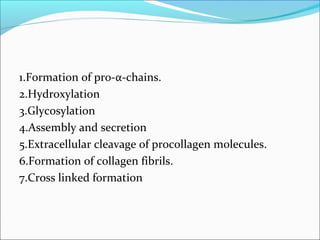
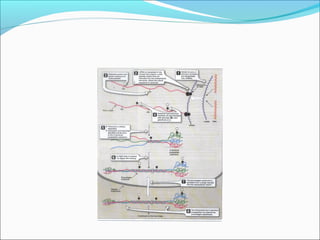
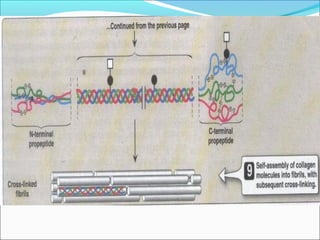
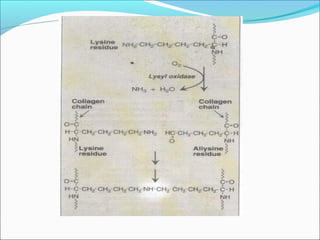
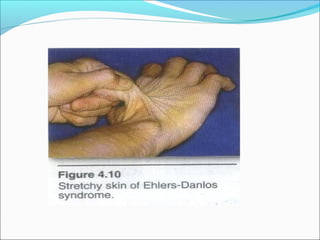
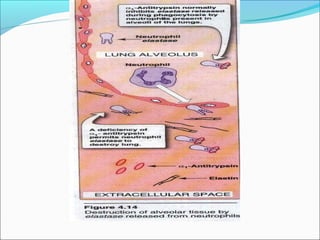
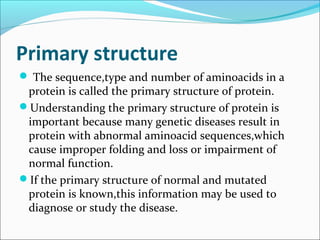
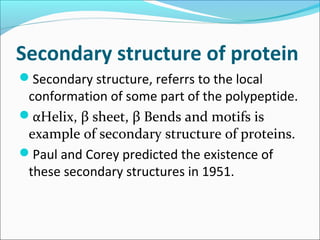
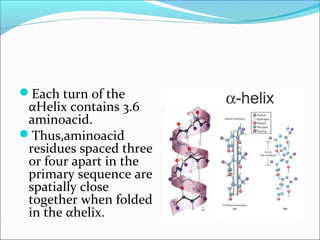
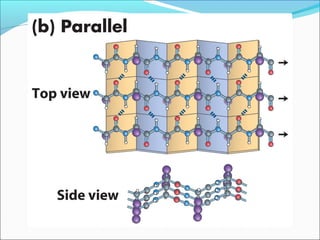
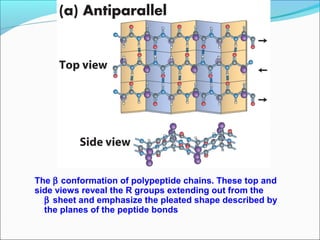
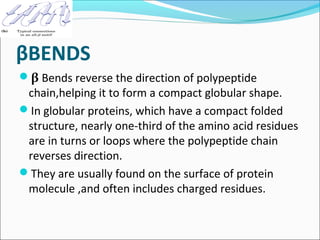
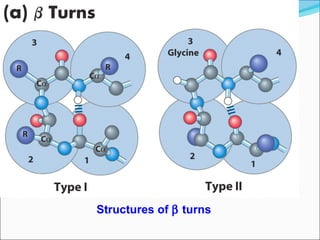
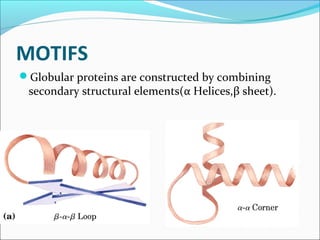
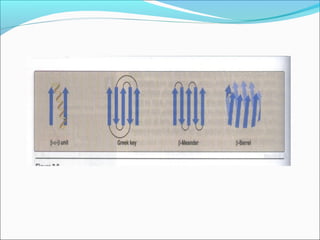
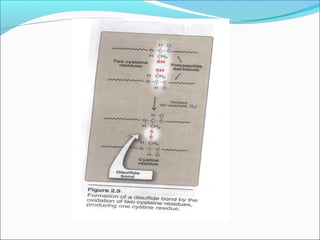
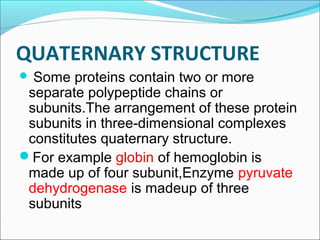
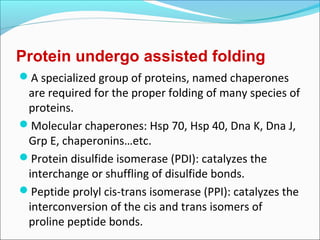
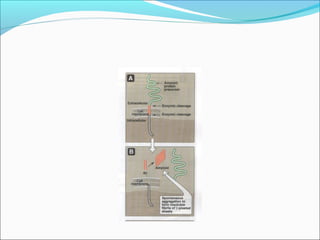
Collagen and elastin are fibrous proteins that provide structure in the body. Collagen forms a rigid triple helix structure made of three polypeptide chains. It is abundant in skin, bone, and cartilage. Elastin provides elasticity and is found in lungs, arteries, and ligaments. Both proteins derive their mechanical properties from their unique secondary and tertiary structures, which are stabilized by interactions between amino acid side chains.