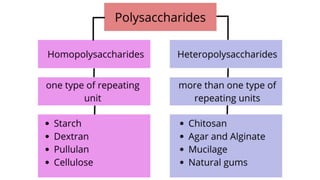
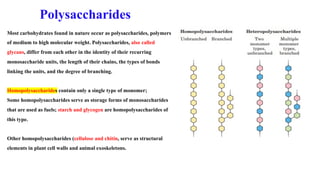
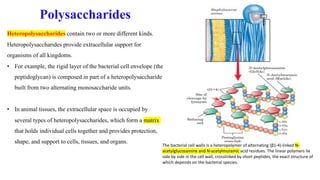
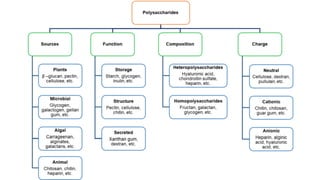
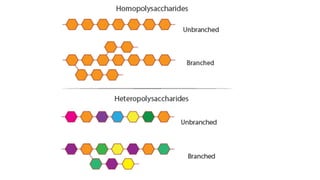
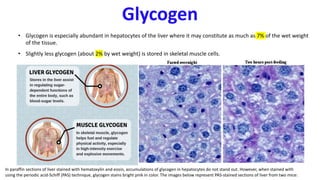
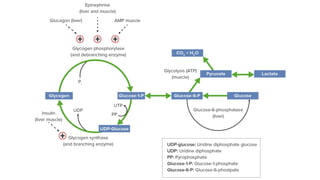
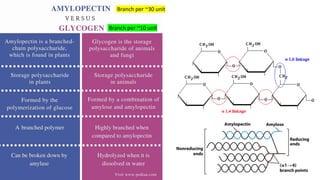
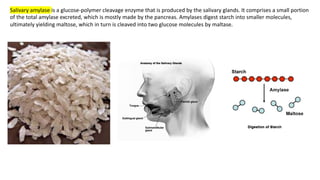
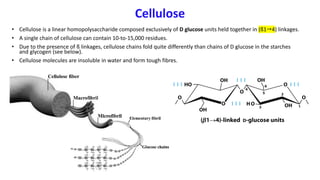
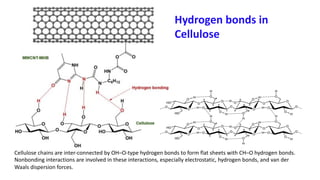
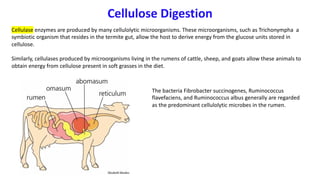
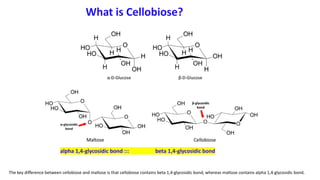
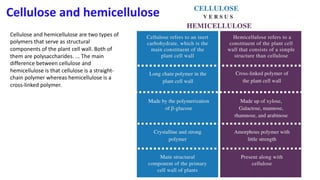
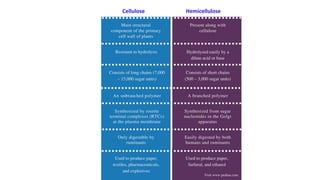
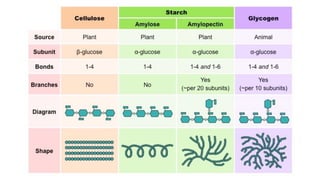
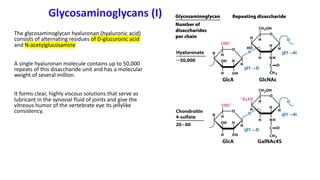
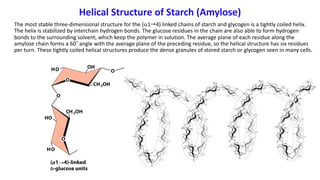
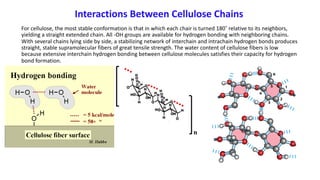
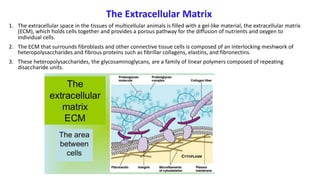
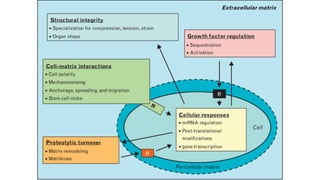
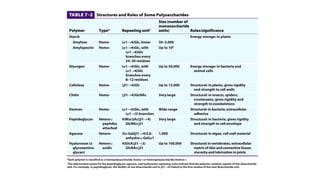
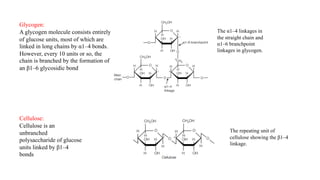
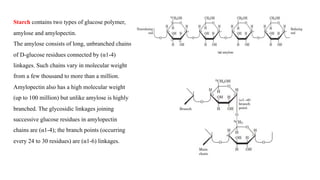
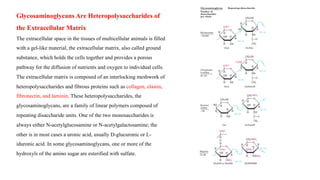
The document discusses polysaccharides, identifying both homopolysaccharides, like starch and glycogen, which serve as energy storage, and heteropolysaccharides, which provide structural support in various organisms. It highlights the differences in structure and function among these polysaccharides, including their linkages and roles in cellular integrity and metabolism. Additionally, it covers the significance of enzymes in polysaccharide digestion and their varied applications in biological systems.