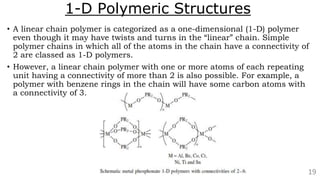
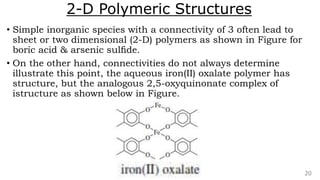
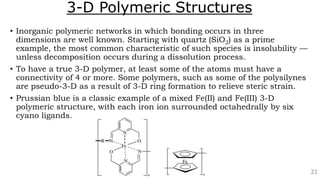
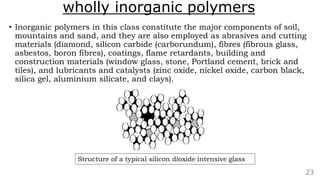
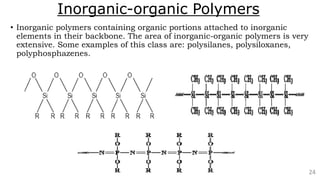
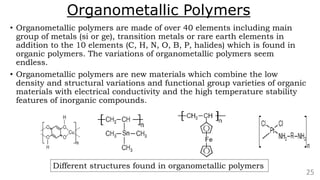
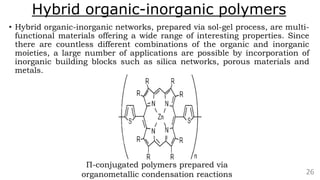
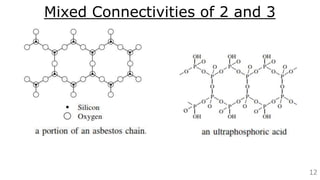
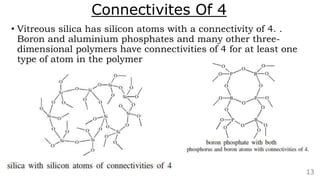
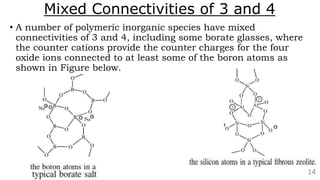
The document discusses the classification of inorganic polymers, highlighting their necessity over organic polymers due to their unique properties such as higher stability and resistance to degradation. It details various classification parameters, including connectivity and dimensionality, as well as the types of inorganic polymers like wholly inorganic, inorganic-organic, organometallic, and hybrid organic-inorganic. Inorganic polymers play significant roles in materials science and have diverse applications across multiple industries.



![Connectivities of 8
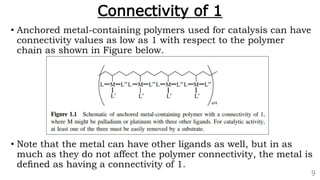
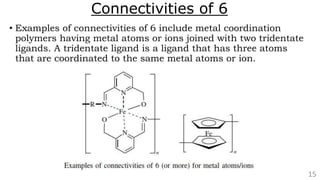
• Metal coordination polymers of zirconium(IV), yttrium(III), and several
lanthanide ions [cerium(IV), lanthanum(III), europium(III), gadolinium(III),
and lutetium(III)] have been synthesized that possess connectivities of 8
because two tetradentate ligands are coordinated to each metal ion that is
part of the polymer chain. An example is shown below in Figure.
17](https://image.slidesharecdn.com/classificationofinorganicpolymers-dev-170305110827/85/Classification-of-inorganic-polymers-17-320.jpg)