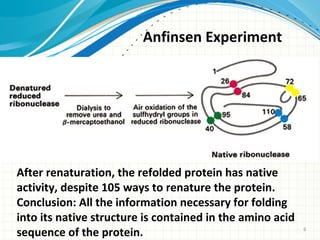
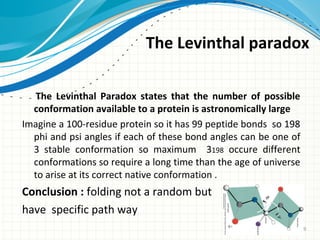
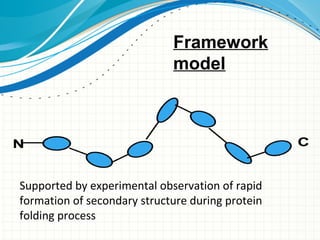
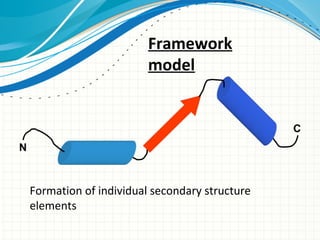
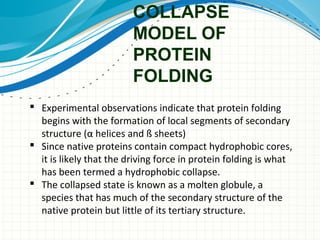
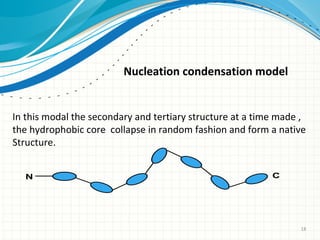
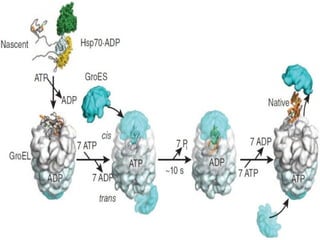
The document discusses protein folding, emphasizing how amino acid sequences dictate a protein's native structure through physical forces and the challenges associated with predicting protein structure and folding mechanisms. It also details Anfinsen's experiments, which demonstrated that proteins can refold to their native state provided the correct conditions, and explains various folding models and the role of molecular chaperones in assisting protein folding. Additionally, it mentions the Levinthal paradox, which illustrates the complexity of protein conformations, and highlights the importance of hydrophobic interactions in the folding process.