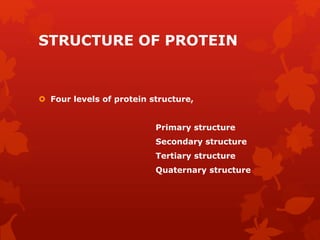
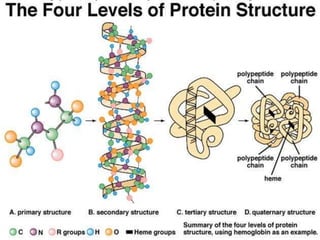
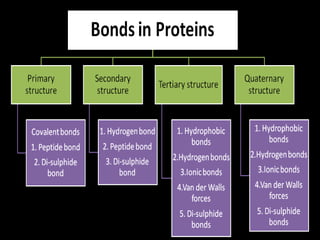
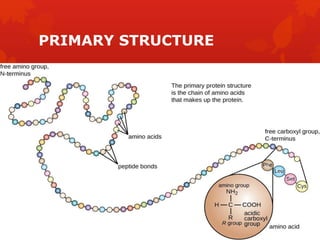
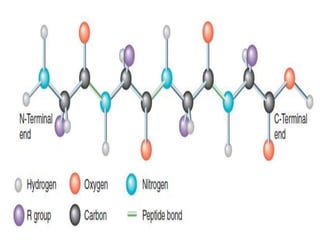
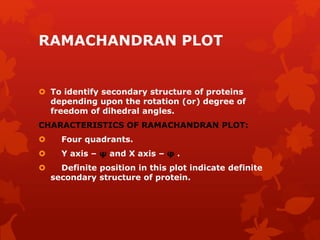
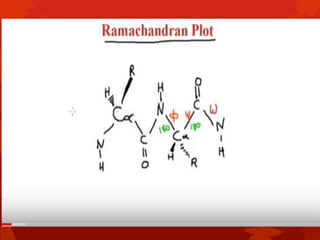
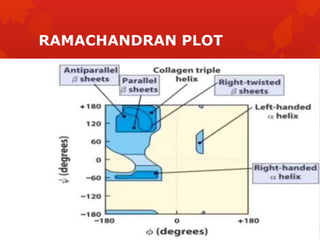
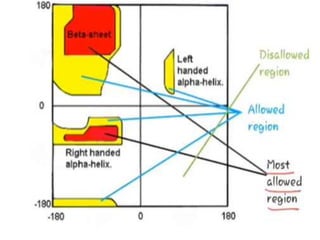
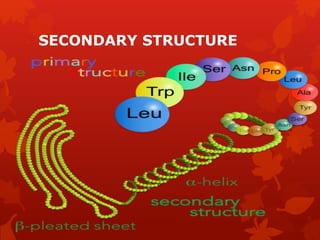
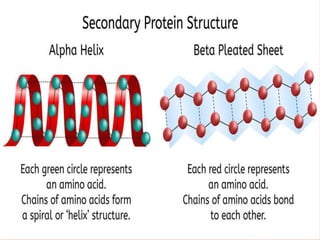
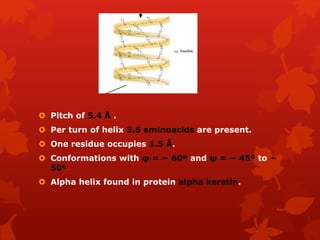
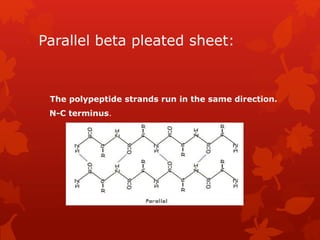
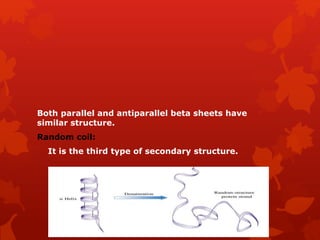
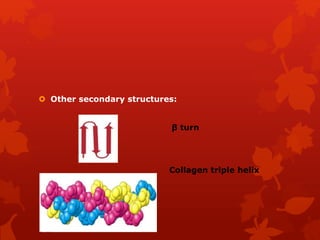
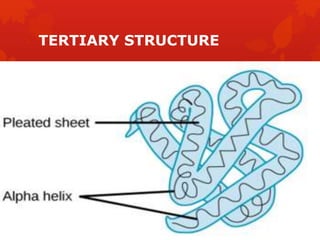
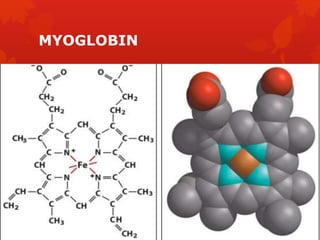
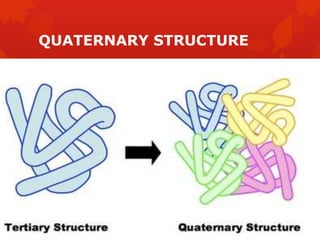
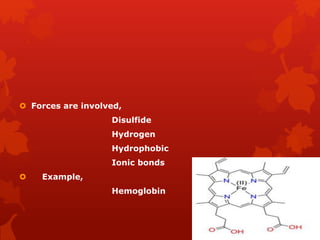
This document summarizes the four levels of protein structure: primary, secondary, tertiary, and quaternary. It provides details on each level: primary structure refers to the linear sequence of amino acids in the polypeptide chain. Secondary structure involves hydrogen bonding between amino acids to form regular structures like alpha helices and beta pleated sheets. Tertiary structure describes the overall 3D shape of the protein arising from secondary structures. Quaternary structure involves interactions between two or more polypeptide chains, as seen in hemoglobin which is made of four polypeptide subunits.