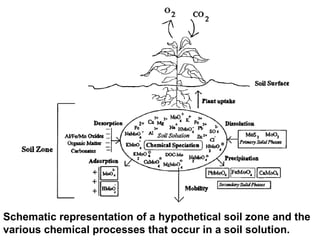
This document provides information about soil fertility and nutrient management. It discusses key concepts like soil fertility, plant nutrients, integrated plant nutrient management, and how physical properties, water, and problem soils impact nutrient management. Organic matter, soil reactions, fertility evaluation methods, and nutrient interactions are also covered. The document lists various topics to be covered in the syllabus like nutrient functions, deficiency diagnosis, and principles of determining macro and micronutrients. It pays tribute to notable scientists who contributed to the field.




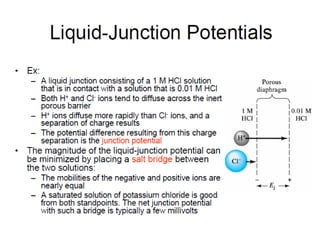
![Thus the product [ H+
][ OH-
] in aqueous solutions at 25°C always equals 1x10 -14
M2
.
When there are exactly equal concentrations of H+
and OH-
, as in pure water, the solution
is said to be at neutral pH. At this pH, the concentration of H+
and OH-
can be calculated
from the ion product of water as follows:](https://image.slidesharecdn.com/acss-451class1forph2016-160908081948/85/pH-and-Soil-pH-6-320.jpg)


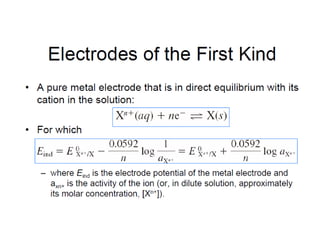
![[ ][ ]
[ ]HAca
K
−+
=
AcH
[ ] [ ]
[ ]-
Ac
H
HAcKa
=+
[ ]HAc
Aclog
a
pKpH
−
+=∴
This form of ionisation constant equation is called the
Henderson- Hasselbalch equation.
pH of an weak acid
[ ]
−
+= AcHHAc](https://image.slidesharecdn.com/acss-451class1forph2016-160908081948/85/pH-and-Soil-pH-9-320.jpg)

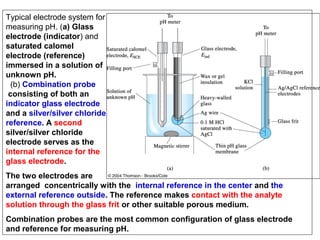

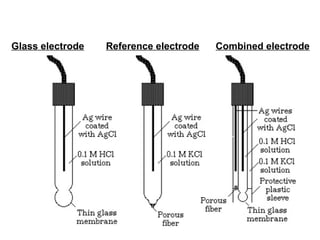
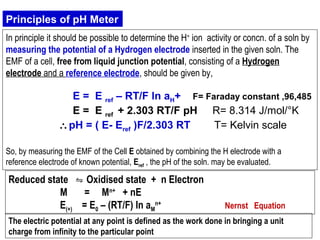





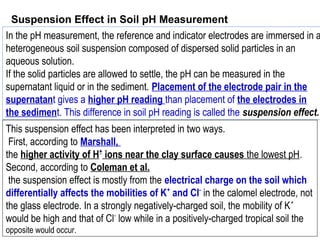













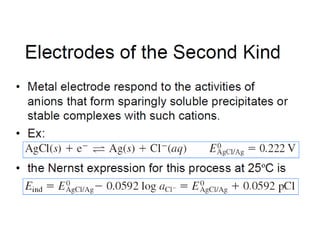
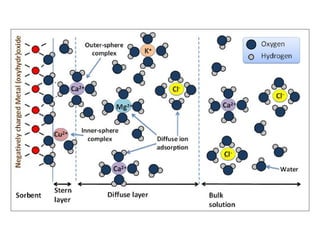
![The ionic distribution at a negatively charged clay
surface.
Gouy- Chapman Model
Assumptions:
The surface is assumed to
be flat, or infinite extent and
uniformly charged.
Ions are assumed to be
point charges distributed as
per Boltzmann distribution
ni =n0
iexp[ -zie0Ψx/κT]
n0
i =no of ions in bulk soln
ni =no of ions at a distance x
from the surface
Ψx = electrical potential
Κ = Boltzmann constant
Zi = valence, e0= charge of electron
Solvent will influence the dl
only through its uniforrn
dielectric constant
The potential decays
exponentially into the soln;
deep enough inside the solution,
x→α, the potential becomes zero](https://image.slidesharecdn.com/acss-451class1forph2016-160908081948/85/pH-and-Soil-pH-35-320.jpg)









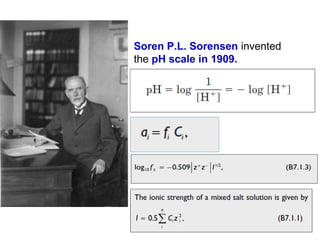
![pH Electrodes
2.) Glass Membrane
Two surfaces of glass “swell” as they absorb water
- Surfaces are in contact with [H+
]](https://image.slidesharecdn.com/acss-451class1forph2016-160908081948/85/pH-and-Soil-pH-46-320.jpg)
![pH Electrodes
2.) Glass Membrane
H+
diffuse into glass membrane and replace Na+
in hydrated gel
region
- Ion-exchange equilibrium
- Selective for H+
because H+
is only ion that binds
significantly to the hydrated gel layer
H(0.05916)constant pE β−=
Charge is slowly carried
by migration of Na+
across glass membrane
Potential is determined
by external [H+
]
Constant and b are measured when electrode is calibrated with solution of known pH](https://image.slidesharecdn.com/acss-451class1forph2016-160908081948/85/pH-and-Soil-pH-47-320.jpg)