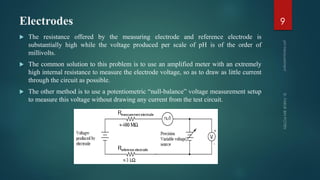
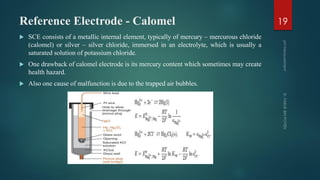
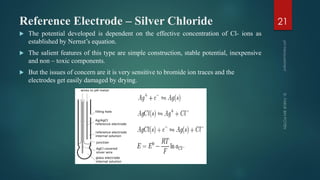
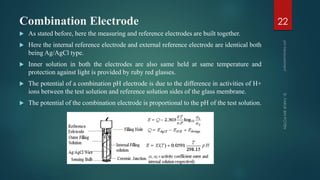
The document discusses pH measurement and the components used. It describes that pH is a measurement of hydrogen ion concentration on a logarithmic scale from 0-14. It also discusses Nernst's equation, which relates electrode potential to ion concentration. The key components used for pH measurement are glass electrodes, reference electrodes like calomel or silver-silver chloride, and buffer solutions. The document provides details on the construction and functioning of these different electrode types.



![pH..
The pH value is expressed as
𝑝𝐻 =
1
𝑙𝑜𝑔10 𝐶
, 𝐶 = 𝑐𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 𝑜𝑓 𝐻+ 𝑖𝑜𝑛𝑠
Solution's pH is measured on its net concentration of hydrogen ions [H+] compared to
concentration of hydroxide ions [OH-].
Acids dissociates to produce H+ ions whereas bases dissociates to produce OH- ions.
The product of these two concentrations of H+ and OH – gives the dissociation
constant pH + pOH = pKW. At 25°C under standard conditions, pH + pOH = 14,
which is why the scale for pH usually ranges from 0 to 14.
4](https://image.slidesharecdn.com/phmeasurement-161121114523/85/pH-Measurement-4-320.jpg)

















![Buffer Solution
Buffer capacity, β, is a quantitative measure of the resistance of a buffer solution to pH
change on addition of hydroxide ions. It can be expressed as
𝛽 =
𝑑𝑛
𝑑(𝑝[𝐻+])
There are three regions of high buffer capacity.
• At very low p [H+] the first term predominates and β increases in proportion to the hydrogen ion
concentration. This is independent of the presence or absence of buffering agents and applies to all
solvents.
• In the region p [H+] = p Ka ± 2 the second term becomes important. Buffer capacity is proportional to
the concentration of the buffering agent, CA, so dilute solutions have little buffer capacity.
• At very high p [H+] the third term predominates and β increases in proportion to the hydroxide ion
concentration. This is due to the self-ionization of water and is independent of the presence or absence
of buffering agents.
26
d n = infinitesimal amount of added base;
d (p[H+]) = resulting infinitesimal change in the co logarithm of the hydrogen ion concentration.
Buffer capacity for a 0.1 M solution of an acid with p Ka of 7](https://image.slidesharecdn.com/phmeasurement-161121114523/85/pH-Measurement-26-320.jpg)






