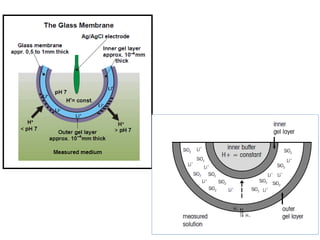
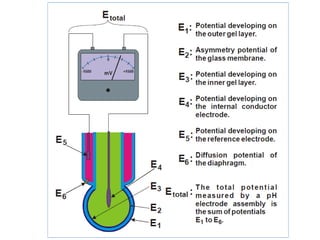
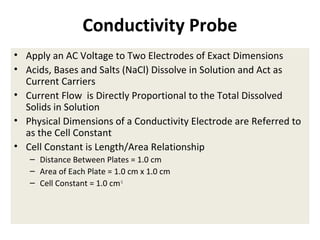
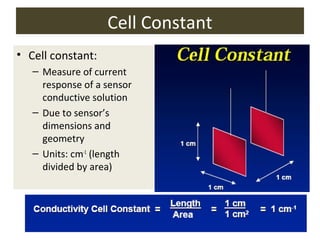
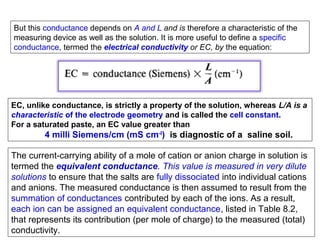
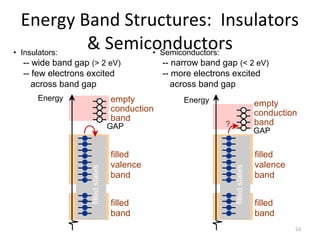
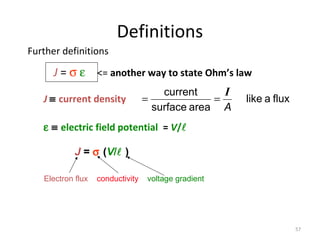
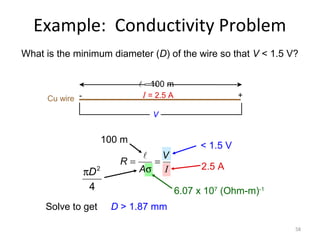
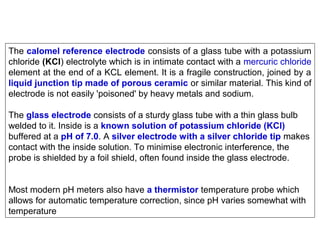
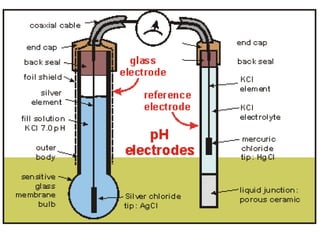
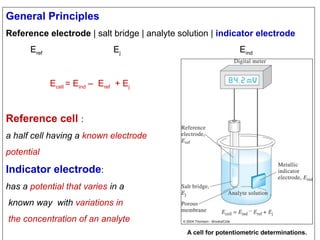
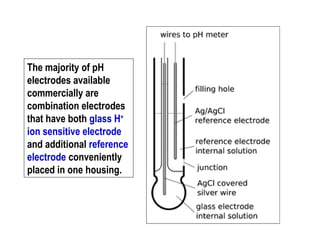
1) A pH meter works by measuring the potential difference between a glass electrode that responds to hydrogen ion concentration and a reference electrode with a known potential. The glass electrode selectively binds hydrogen ions, generating a potential based on the H+ concentration difference across the membrane.
2) The Nernst equation relates the measured potential to pH. At room temperature, pH equals the measured potential minus the reference electrode potential, divided by 0.05916 volts per pH unit.
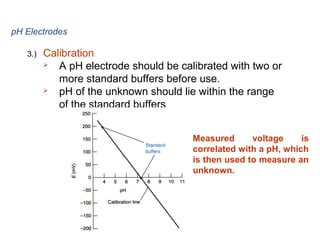
3) Combination pH electrodes contain both the glass and reference electrodes in one probe for convenient measurement of the solution's pH based on its hydrogen ion concentration.

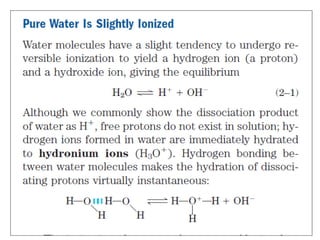
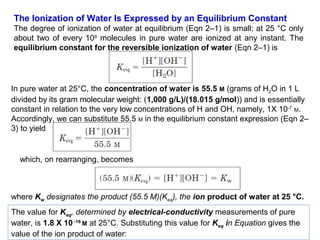
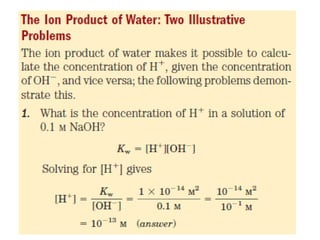
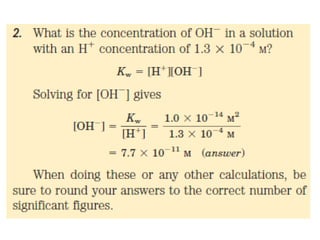
![Thus the product [ H+ ][ OH- ] in aqueous solutions at 25°C always equals 1x10 -14 M2. When
there are exactly equal concentrations of H+ and OH-, as in pure water, the solution is said to
be at neutral pH. At this pH, the concentration of H+ and OH- can be calculated from the ion
product of water as follows:](https://image.slidesharecdn.com/soilphandec-140108022943-phpapp01/85/Soil-pH-and-EC-P-K-MANI-5-320.jpg)

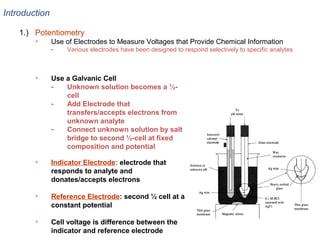
![Reference Electrodes
1.) Overview
Potential change only dependent on one ½ cell
concentrations
Reference electrode is fixed or saturated doesn’t change!
{
[ Fe 2 + ]
0.05916
− 0.222 − 0.05916 log[ Cl − ]
E cell = 0.771 −
log
[ Fe 3 + ]
1
Potential of the cell
only depends on
[Fe2+] & [Fe3+]
Unknown solution of
[Fe2+] & [Fe3+]
}
Reference electrode,
[Cl-] is constant
Pt wire is indicator
electrode whose
potential responds
to [Fe2+]/[Fe3+]](https://image.slidesharecdn.com/soilphandec-140108022943-phpapp01/85/Soil-pH-and-EC-P-K-MANI-12-320.jpg)
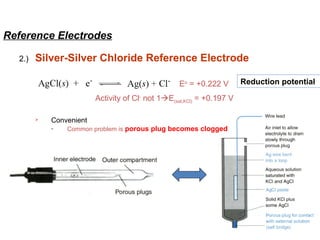
![Reference Electrodes
3.)
Saturated Calomel Reference Electrode (S.C.E)
Eo = +0.268 V
Activity of Cl- not 1E(sat,KCl) = +0.241 V
Saturated KCl maintains constant [Cl-]
even with some evaporation
Standard hydrogen electrodes are
cumbersome
- Requires H2 gas and
freshly prepared Pt surface](https://image.slidesharecdn.com/soilphandec-140108022943-phpapp01/85/Soil-pH-and-EC-P-K-MANI-14-320.jpg)
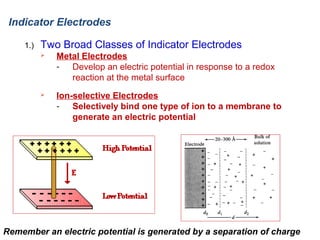
![Indicator Electrodes
2.)
Metal Electrodes
Platinum
Most common metal indicator electrode
Inert: does not participate in many chemical reactions
Simply used to transmit electrons
Other electrodes include Gold and Carbon
Metals (Ag, Cu, Zn, Cd, Hg) can be used to monitor their aqueous ions
Most metals are not useable
Equilibrium not readily established at the metal surface
Example:
E+o = +799 V
½ Reaction at Ag indicator electrode:
E(sat,KCl) = +0.241 V
½ Reaction at Calomel reference electrode:
Cell Potential from Nernst Equation:
1
0.05916
− { 0.241}
E cell = E + − E − = 0.799 −
log
[ Ag + ]
1
Cell voltage changes as a function of [Ag +]
Potential of Ag
indicator electrode](https://image.slidesharecdn.com/soilphandec-140108022943-phpapp01/85/Soil-pH-and-EC-P-K-MANI-16-320.jpg)
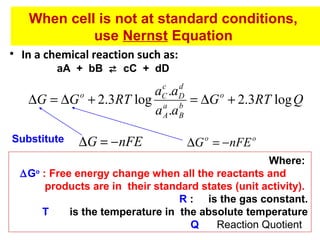
![c
d
aC .aD
∆G = ∆G + 2.3RT log a b
a A .aB
o
[C ]c [ D]d
− nFE = −nFE o + 2.3RT log
[ A]a [ B]b
2.3RT
[C ]c [ D]d
E = Eo −
log
nF
[ A]a [ B]b
Where concentrations are substituted for activities
At 298 K the equation becomes
K
0.0591
[C ]c [ D]d
E = Eo −
log
n
[ A]a [ B]b
…… Nernst Equation
At Equilibrium, ∆G = 0, E = 0. Hence
0.0591
0=E −
log K
n
o
0.0591
E =
log K
n
o](https://image.slidesharecdn.com/soilphandec-140108022943-phpapp01/85/Soil-pH-and-EC-P-K-MANI-20-320.jpg)
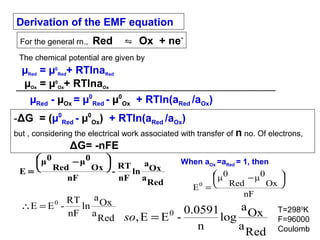
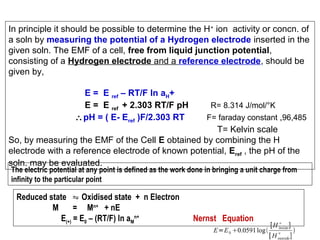
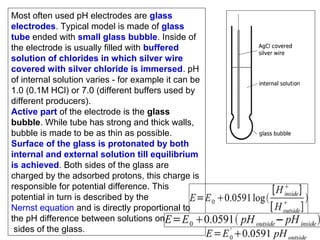
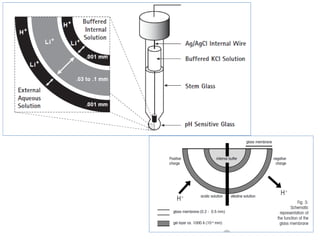
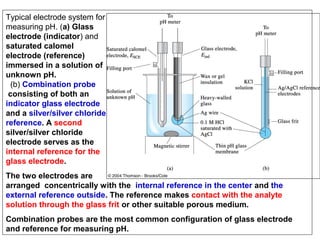
![Electrodes and Potentiometry :
pH Electrodes
1.) pH Measurement with a Glass Electrode
Glass electrode is most common ion-selective electrode
Combination electrode incorporates both glass and
reference electrode in one body
Ag(s)|AgCl(s)|Cl-(aq)||H+(aq,outside) H+(aq,inside),Cl-(aq)|AgCl(s)|Ag(s)
Outer reference
electrode
[H+] outside
(analyte solution)
[H+] inside
Inner reference
electrode
Glass membrane
Selectively binds H+
Electric potential is generated by [H+] difference across glass membrane](https://image.slidesharecdn.com/soilphandec-140108022943-phpapp01/85/Soil-pH-and-EC-P-K-MANI-23-320.jpg)
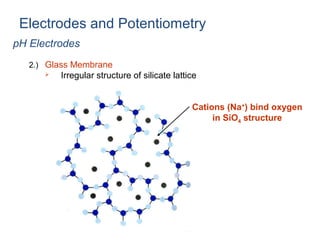
![pH Electrodes
2.) Glass Membrane
Two surfaces of glass “swell” as they absorb water
- Surfaces are in contact with [H+]](https://image.slidesharecdn.com/soilphandec-140108022943-phpapp01/85/Soil-pH-and-EC-P-K-MANI-25-320.jpg)
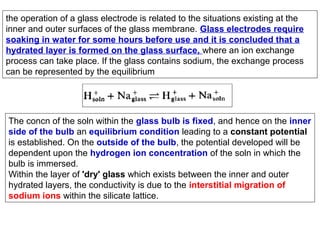
![pH Electrodes
2.) Glass Membrane
H+ diffuse into glass membrane and replace Na+ in hydrated gel
region
-
Ion-exchange equilibrium
Selective for H+ because H+ is only ion that binds
significantly to the hydrated gel layer
Charge is slowly carried
by migration of Na+
across glass membrane
E = constant − β (0.05916) pH
Potential is determined
by external [H+]
Constant and b are measured when electrode is calibrated with solution of known pH](https://image.slidesharecdn.com/soilphandec-140108022943-phpapp01/85/Soil-pH-and-EC-P-K-MANI-26-320.jpg)