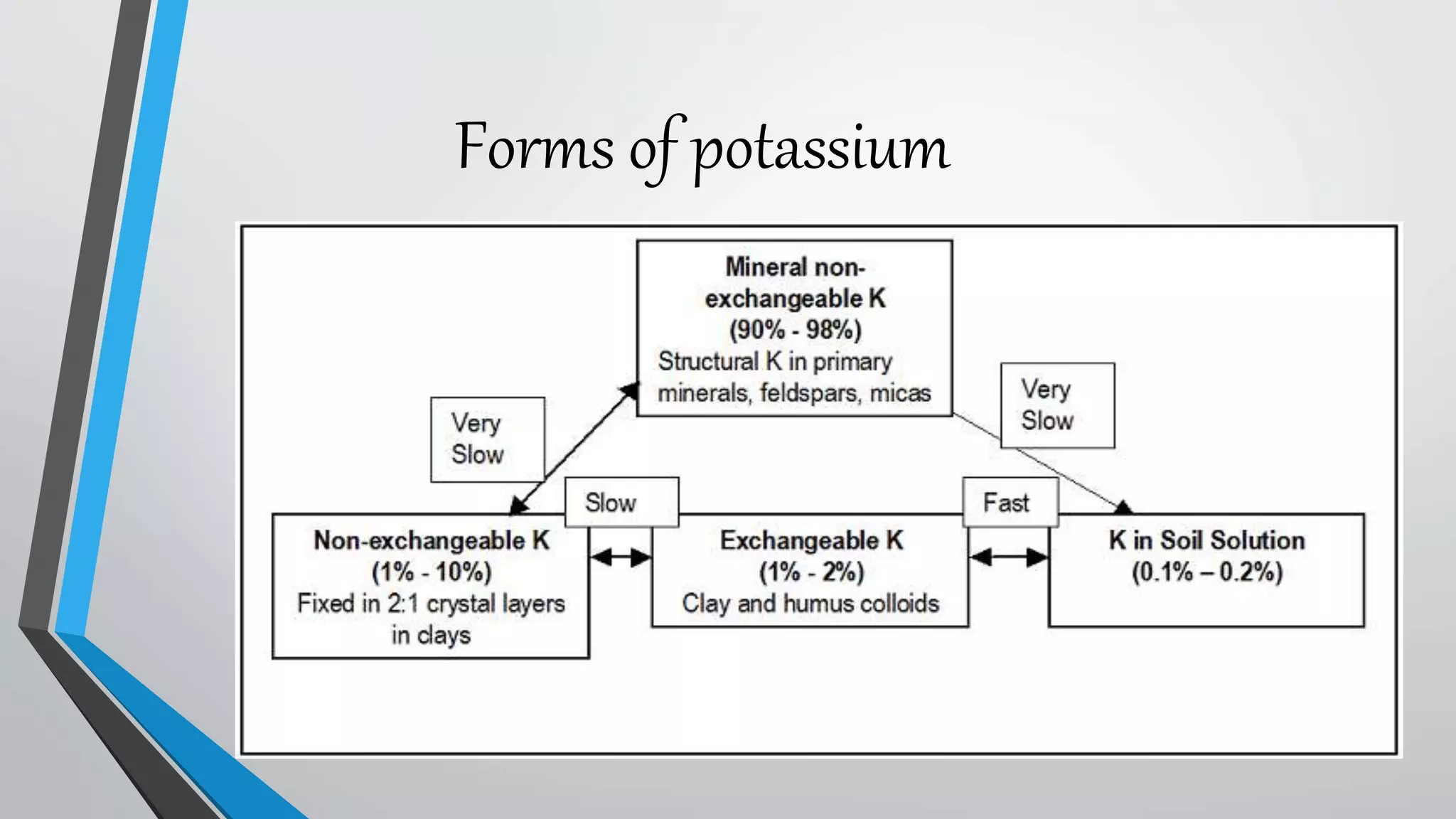
This document discusses potassium fixation in different clay minerals. It explains that potassium fixation was first reported in 1887 and involves potassium penetrating between clay layers and becoming tightly held. The degree of potassium fixation varies between clay types, with vermiculite showing the highest fixation due to its high charge density and large interlayer space, followed by illite, montmorillonite, and kaolinite. Factors like charge density, interlayer space size, solution concentration, and presence of other cations can influence how much potassium is fixed within clay minerals. The practical implication is that fixed potassium contributes to long-term potassium availability in soils.