This document describes several methods for quantitatively analyzing the components of organic compounds:
1. Carbon and hydrogen are estimated by burning the compound in oxygen and measuring the amount of water and carbon dioxide produced.
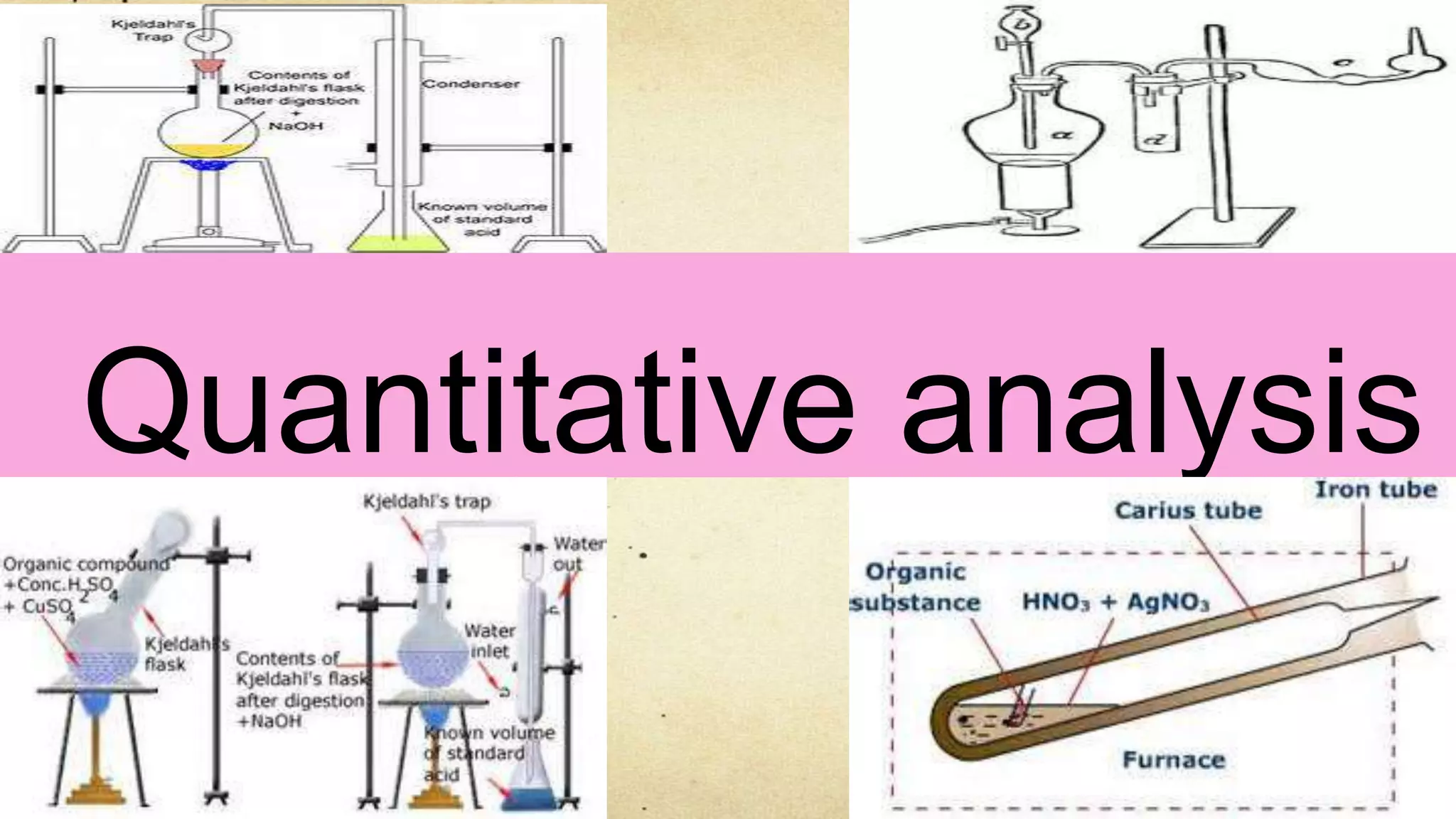
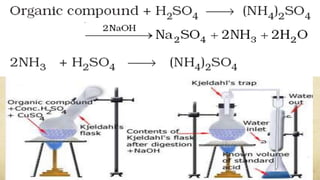
2. Nitrogen can be estimated using the Dumas or Kjeldahl methods, which involve heating the compound to produce nitrogen gas or ammonia for measurement.
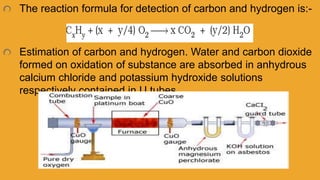
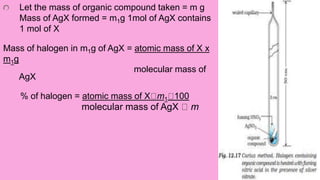
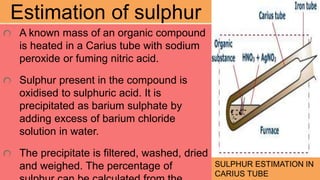
3. Other elements like halogens, sulfur, phosphorus, and oxygen can be estimated through various oxidation and precipitation reactions to form compounds that can be weighed or titrated. Formulas are provided for calculating the percentage of each element from the experimental results.