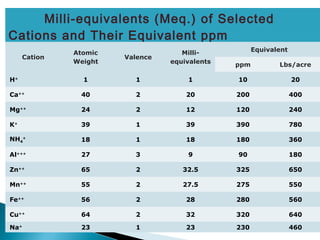
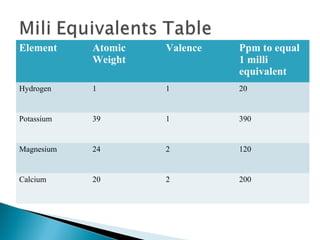
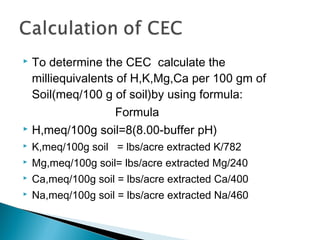
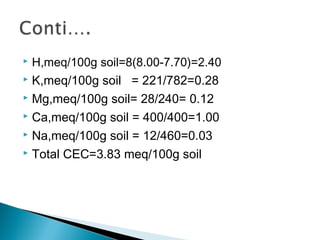
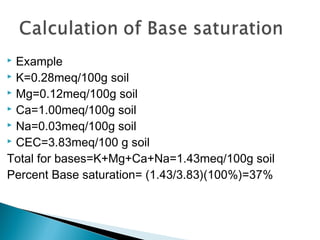
The document discusses plant nutrients, particularly focusing on cations and anions, and their role in soil chemistry and fertility. It emphasizes the importance of cation exchange capacity (CEC) in measuring how well soils can hold exchangeable cations and provides formulas for calculating various soil nutrients. Additionally, the document explains how cation saturation affects soil pH and overall quality, linking cation presence with soil structure and organic matter content.