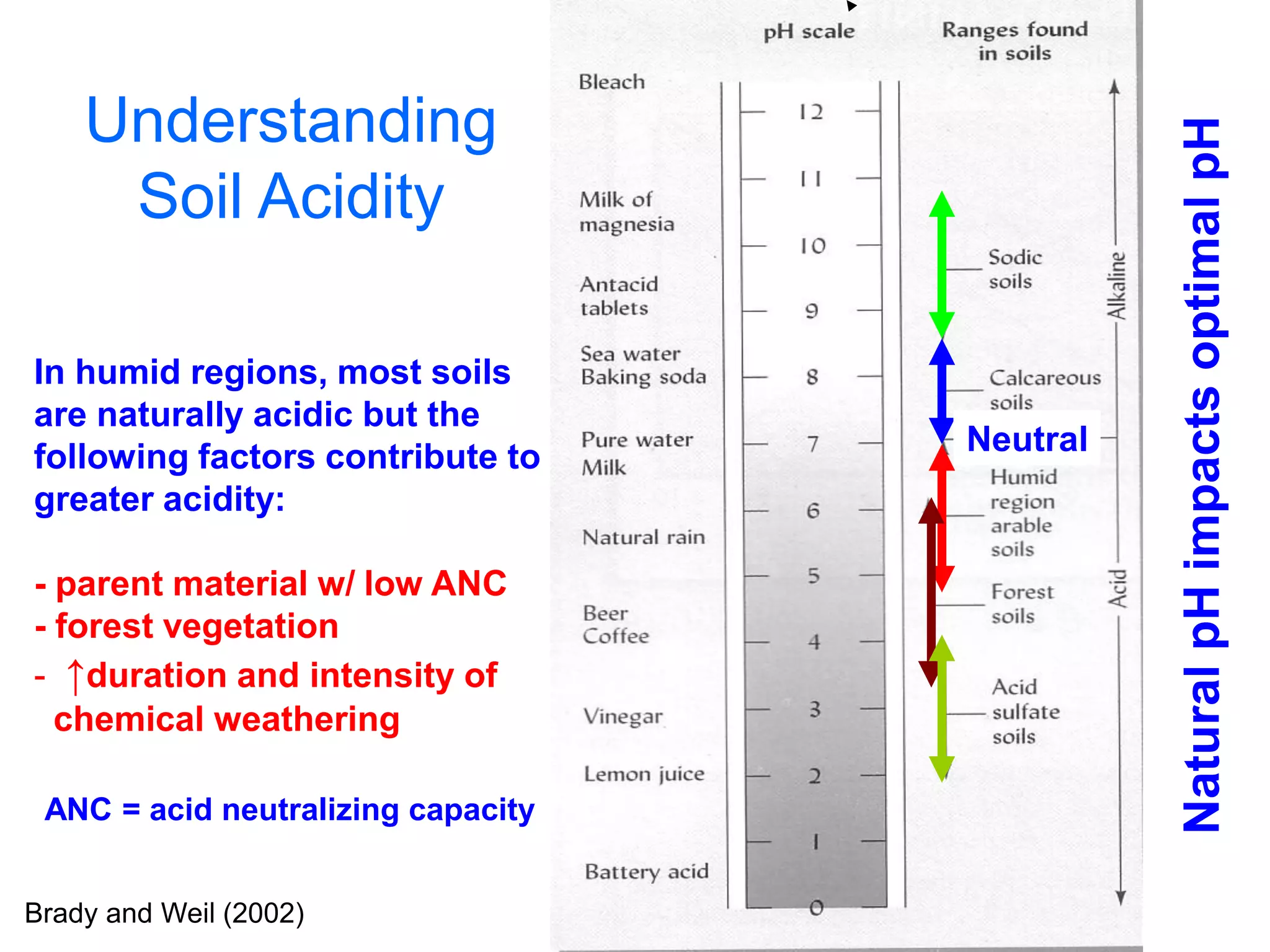
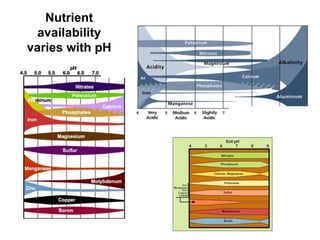
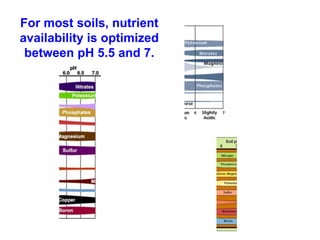
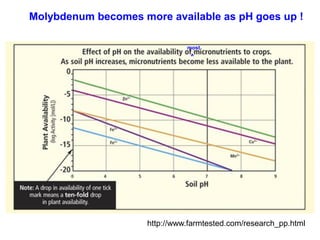
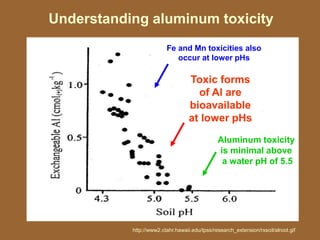
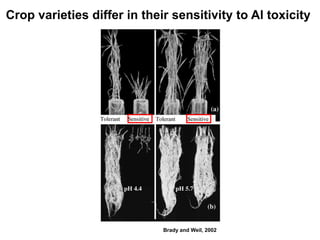
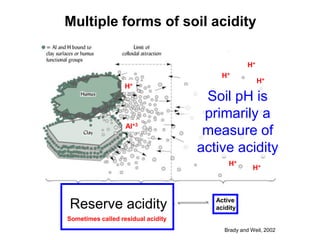
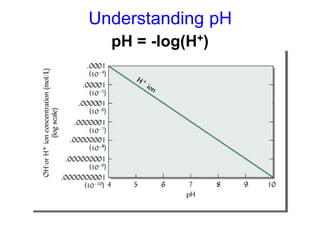
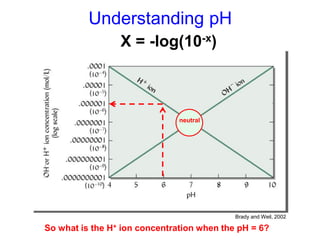
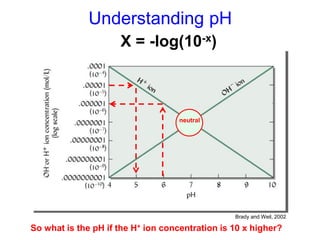
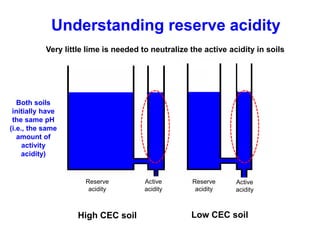
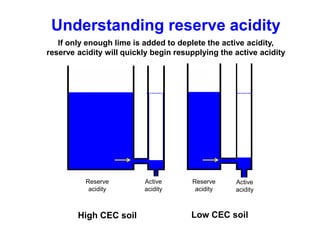
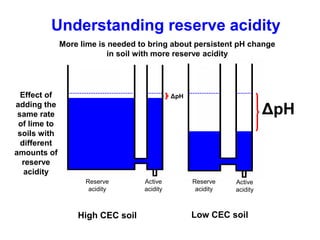
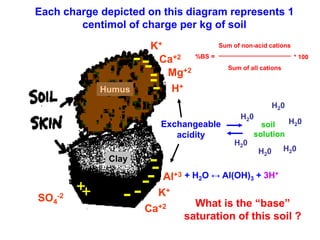
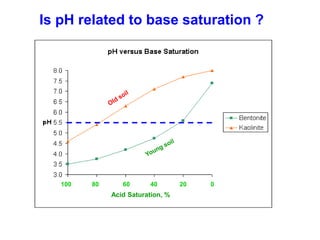
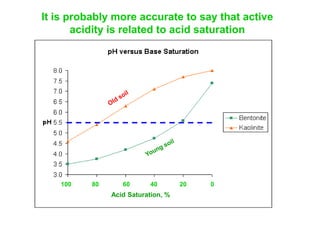
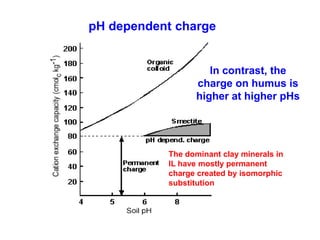
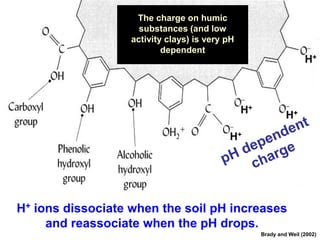
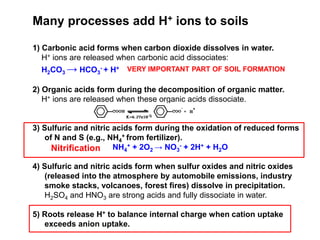
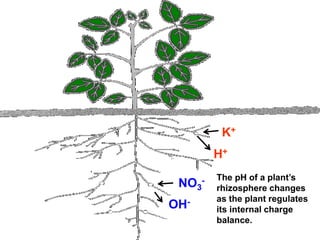
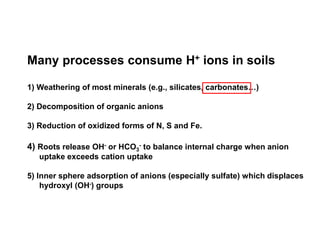
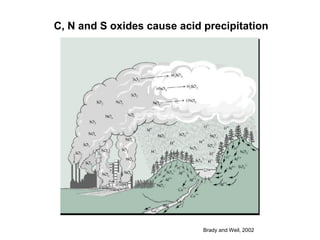
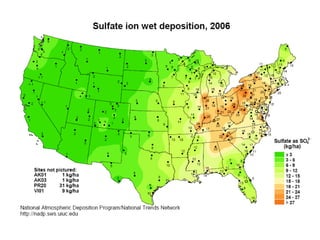
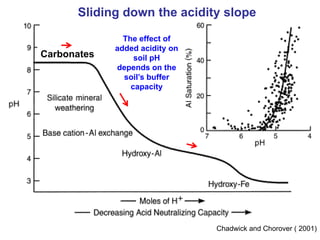
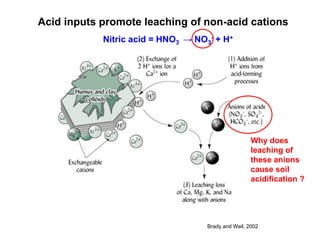
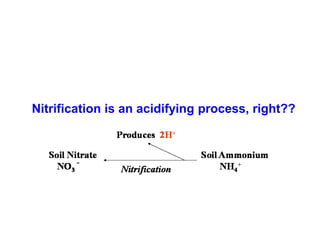
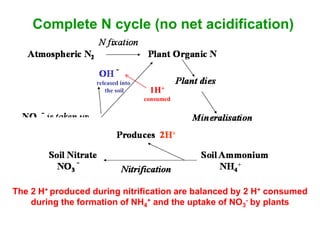
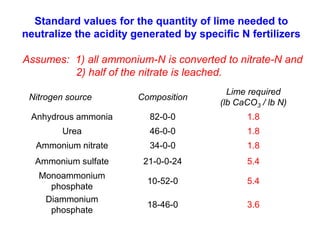
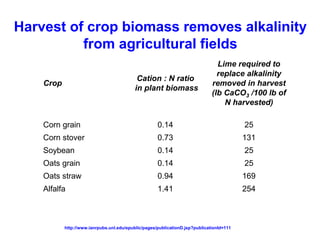
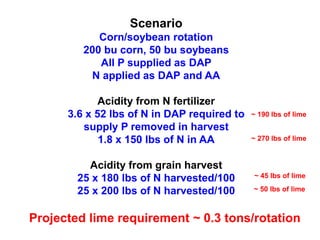
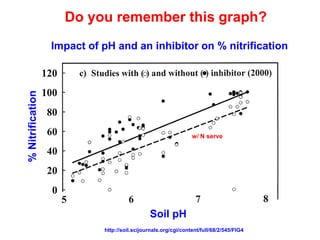
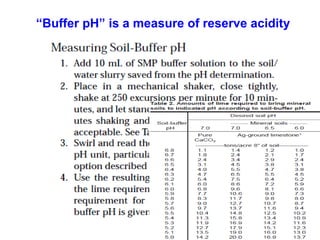
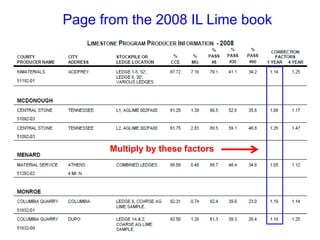
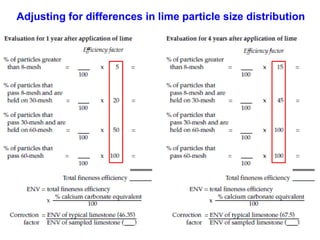
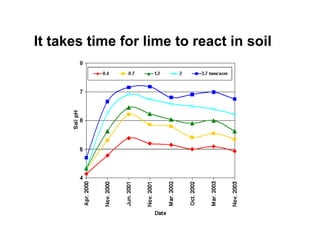
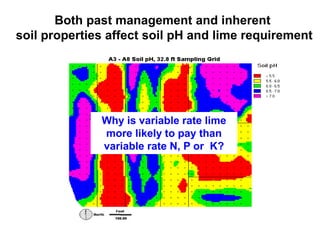
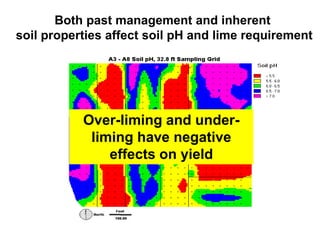
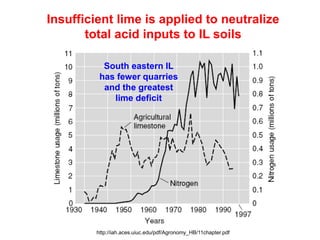
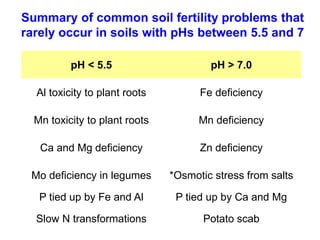
This document discusses soil acidity and pH. It begins by explaining how various natural and anthropogenic factors can contribute to soil acidity in humid regions. It then discusses how pH impacts nutrient availability and toxicity, with most nutrients being optimally available between pH 5.5-7. It also covers aluminum toxicity, how it is more prevalent at lower pH, and how different crop varieties have varying sensitivities. The document provides an overview of the multiple forms of soil acidity and explains pH in terms of hydrogen ion concentration.