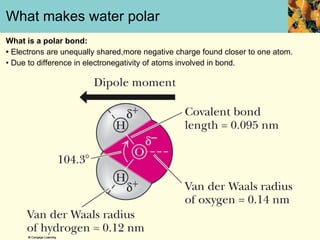
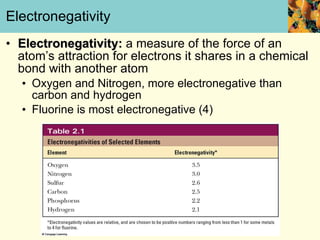
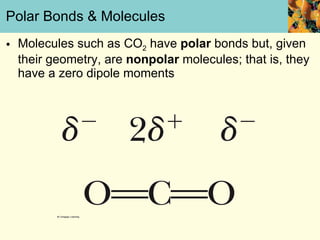
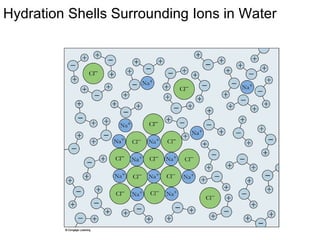
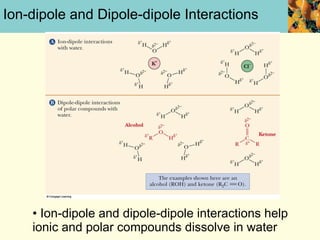
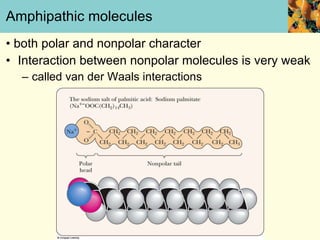
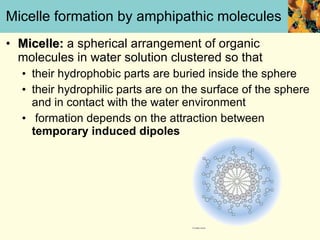
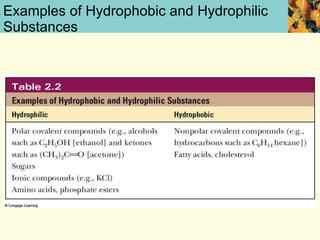
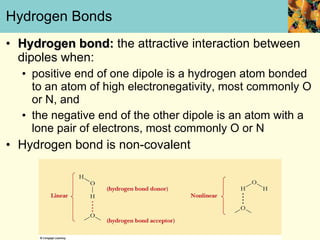
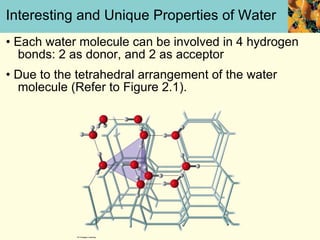
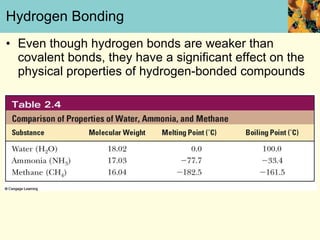
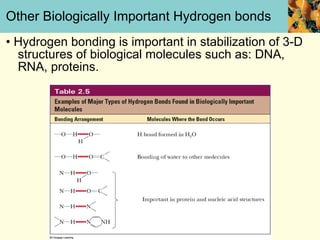
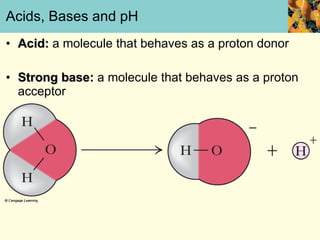
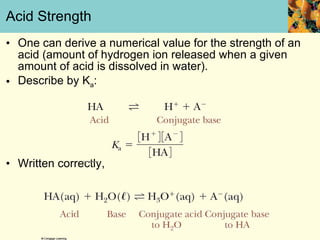
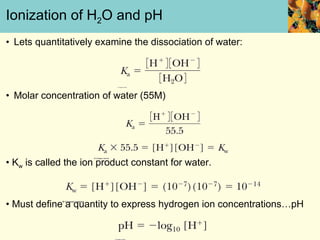
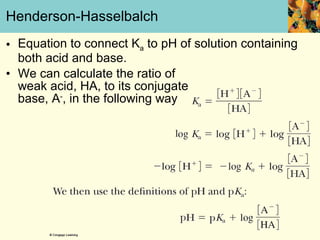
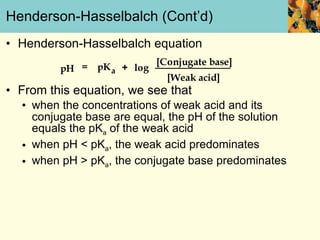
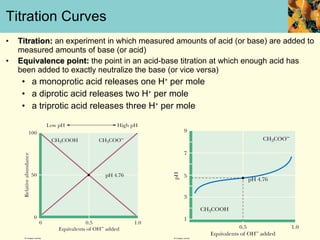
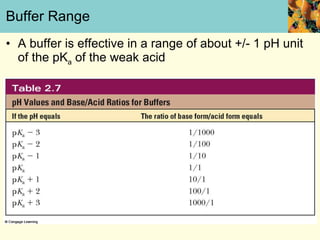
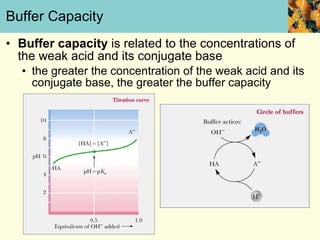
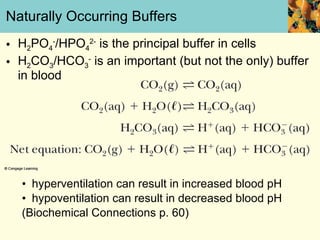
This document discusses several key properties of water that make it an excellent solvent for biochemical reactions. It explains that water is polar due to polar bonds between hydrogen and oxygen atoms. This polarity allows water to interact with ionic compounds and polar molecules through ion-dipole and dipole-dipole interactions, dissolving them. Water also forms hydrogen bonds that contribute to its unique solvent properties and the structure of biological molecules like DNA and proteins.