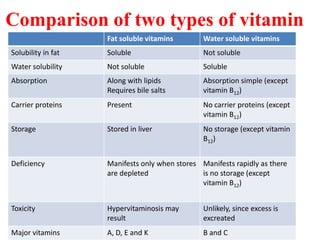
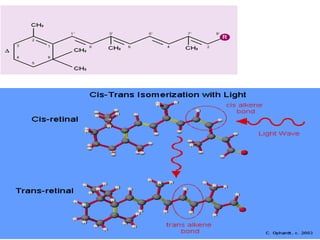
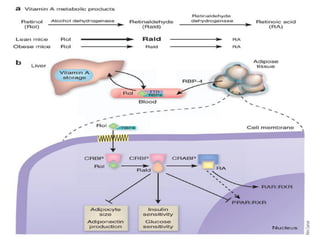
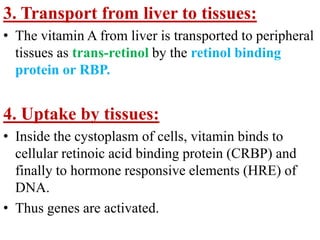
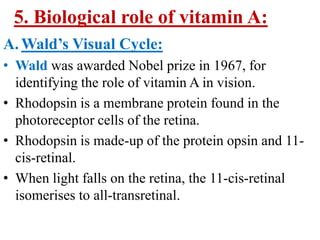
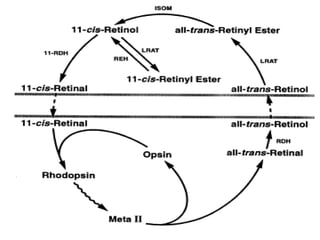
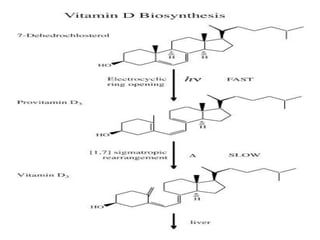
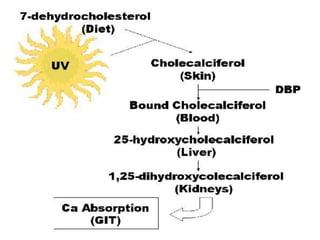
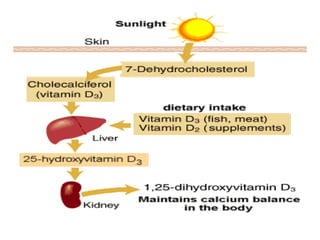
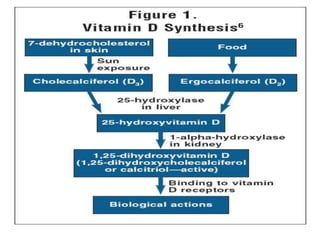
This document discusses fat soluble vitamins, including Vitamins A, D, E, and K. It provides details on the chemical structure, absorption, transport, functions, sources, and requirements of each vitamin. The key roles of Vitamin A are in vision and tissue growth/differentiation. Vitamin D helps absorb calcium and phosphate to support bone mineralization. Vitamins E and K act as antioxidants and are necessary for blood clotting, respectively. A diet containing foods like fish liver, eggs, green vegetables, and plant oils can provide adequate amounts of these essential fat soluble vitamins.