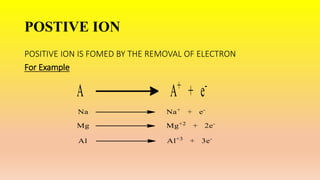
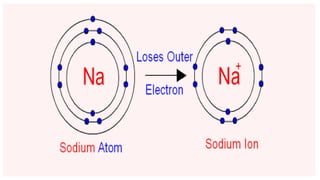
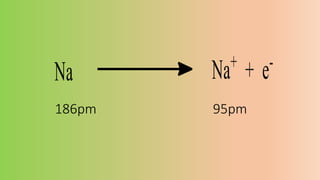
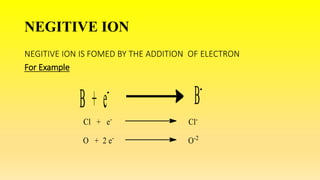
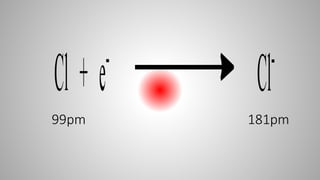
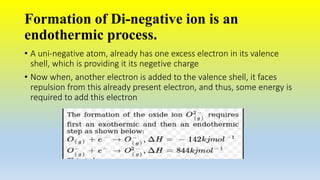
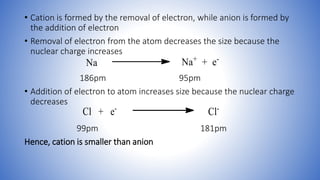
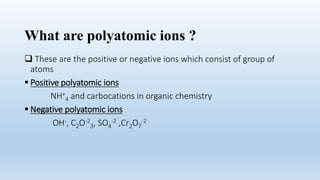
This document defines ions and describes the different types of ions. It states that ions are formed by the addition or removal of electrons from atoms. Positive ions, or cations, are formed when electrons are removed making the atom positively charged. Negative ions, or anions, are formed when electrons are added making the atom negatively charged. Cation formation is an endothermic oxidation reaction while anion formation is an exothermic reduction reaction. The document also explains that cations are smaller than the parent atom, while anions are larger, due to changes in nuclear charge upon electron addition or removal.