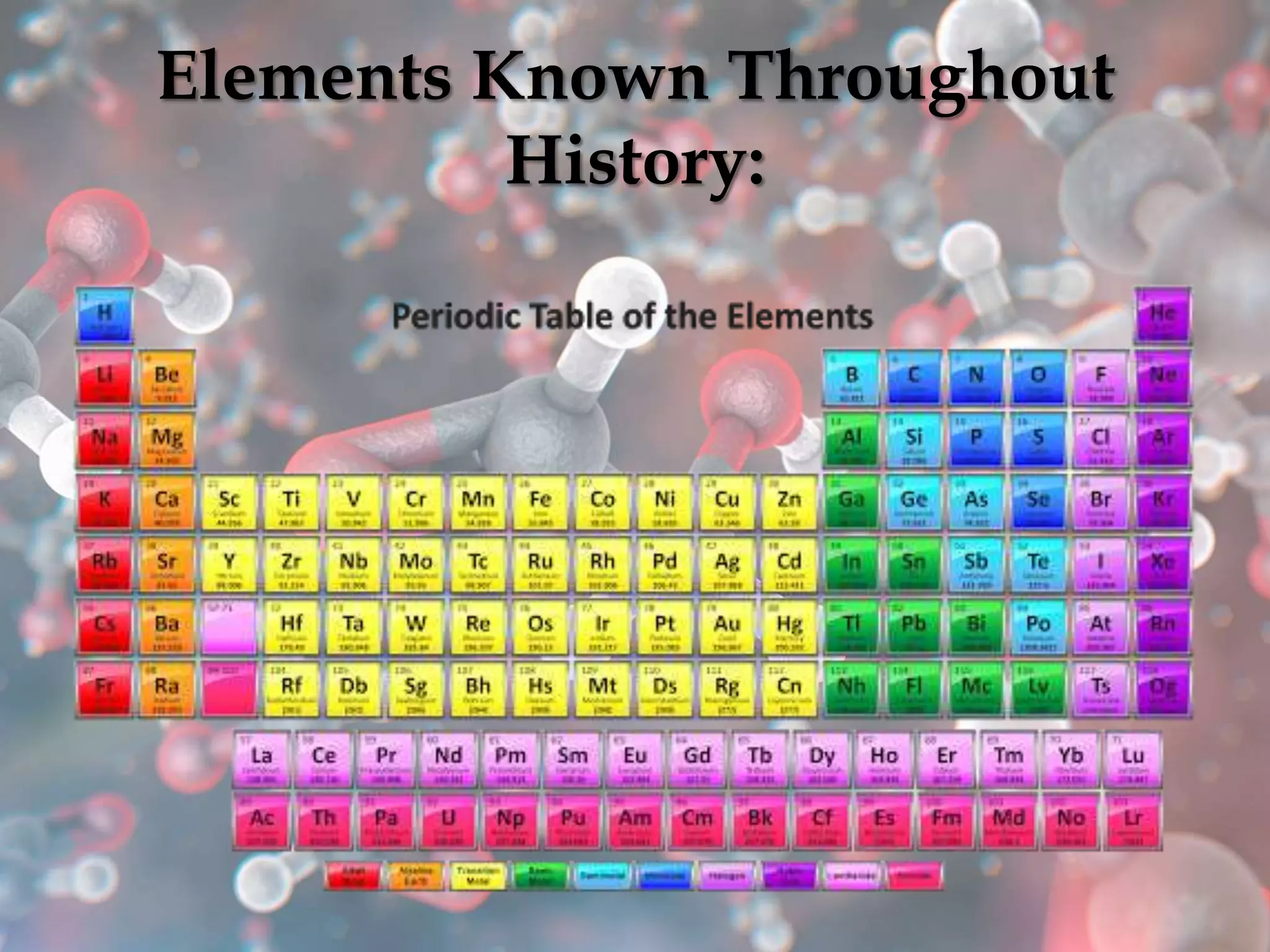
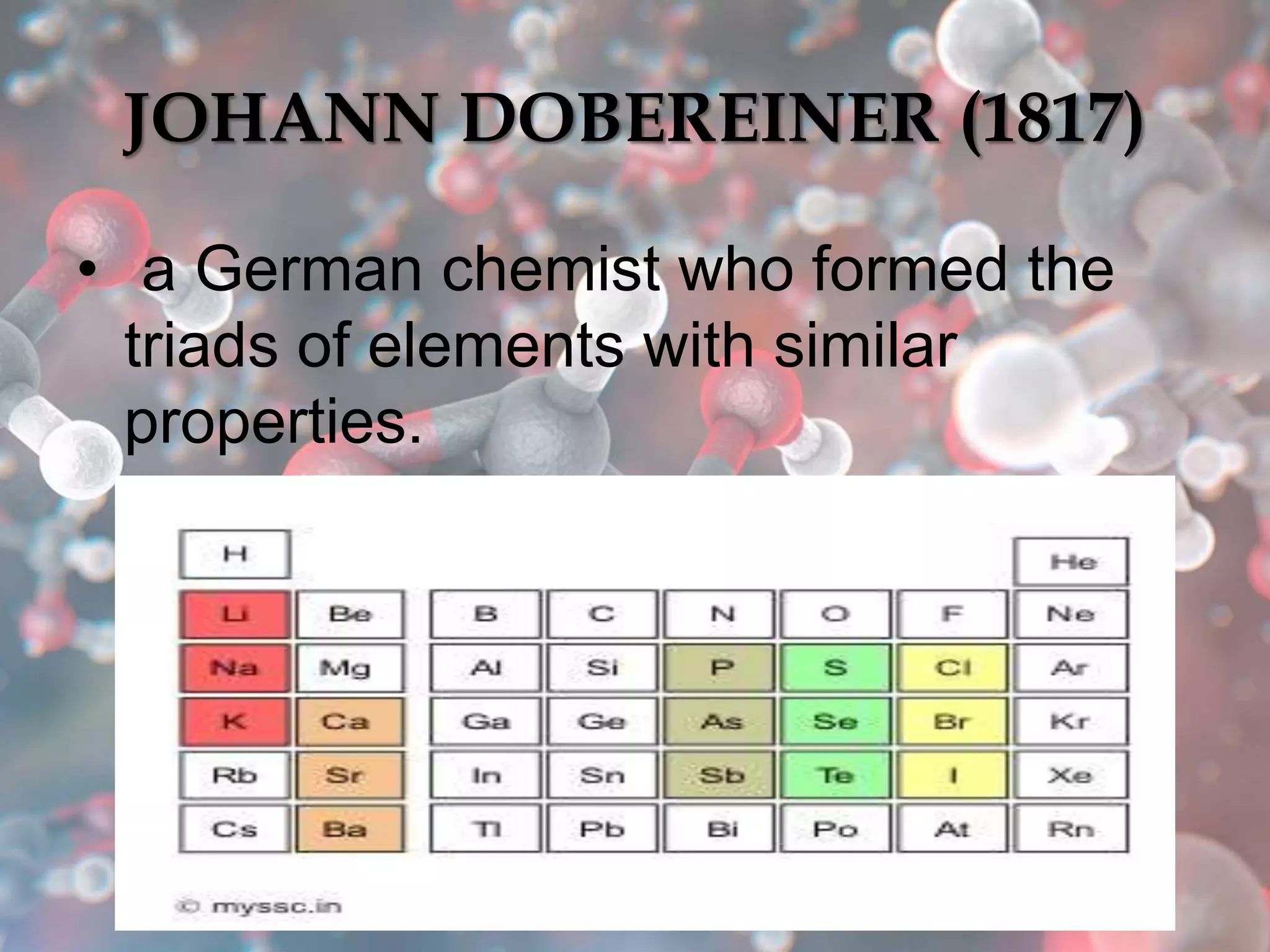
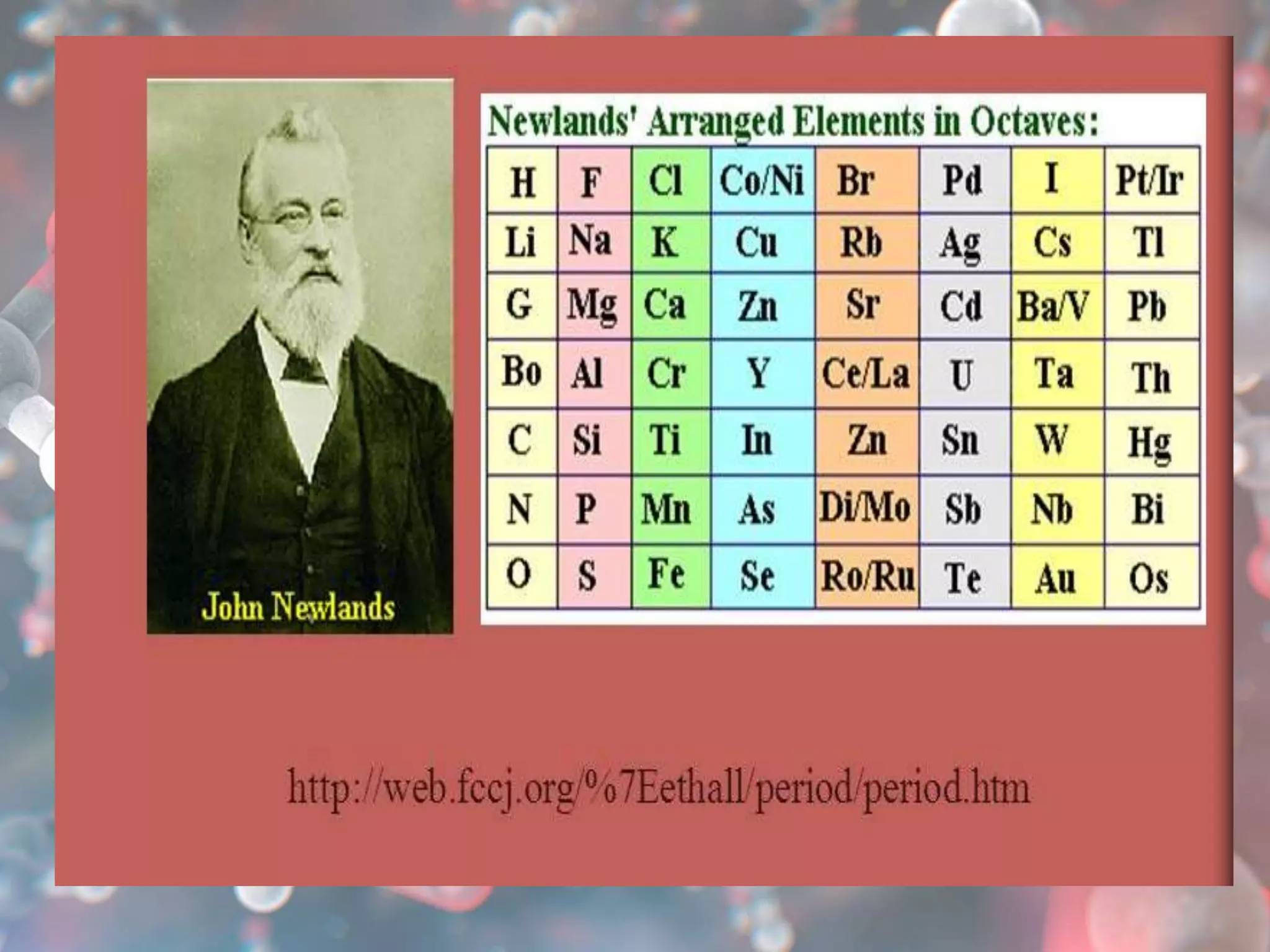
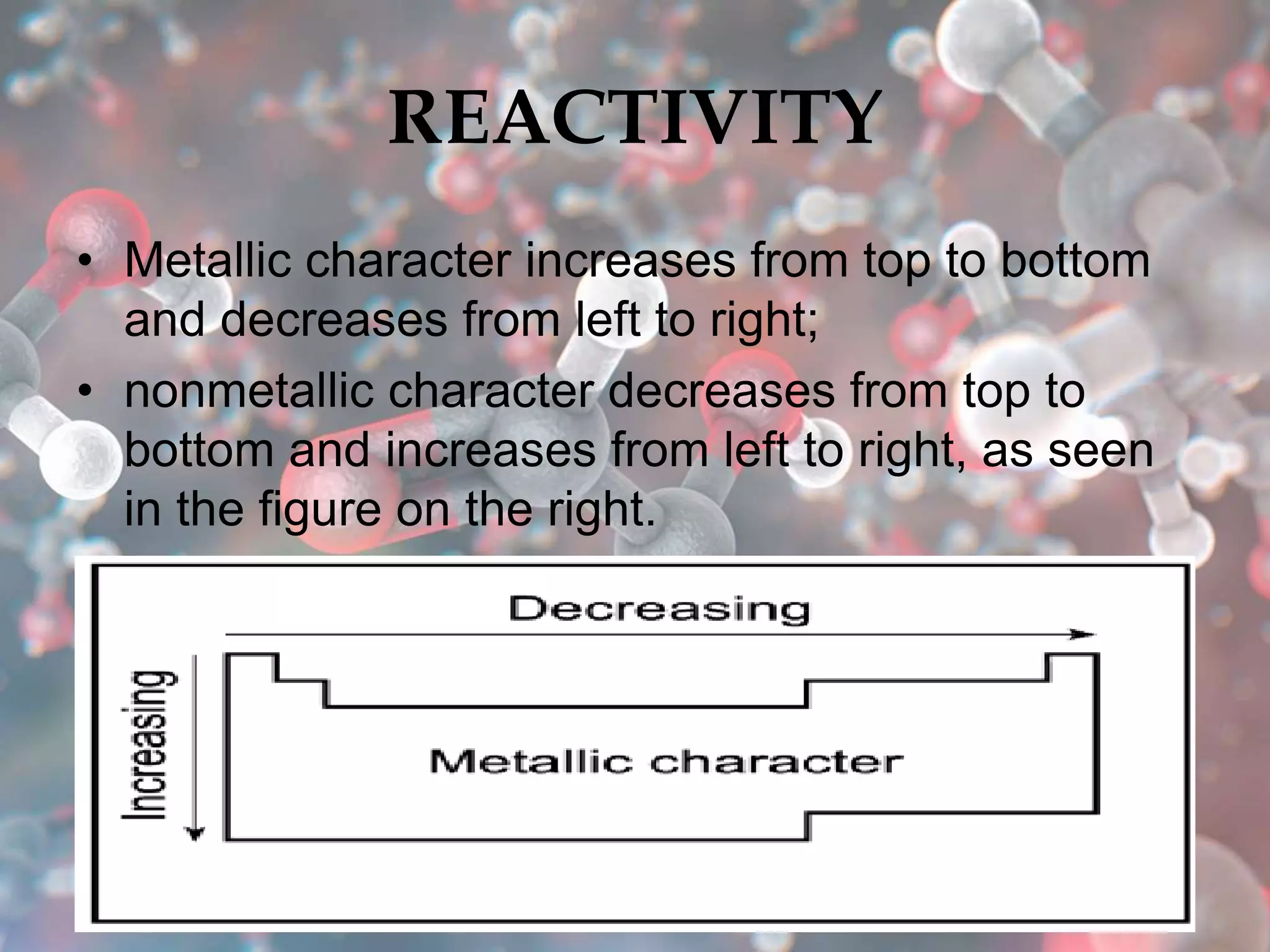
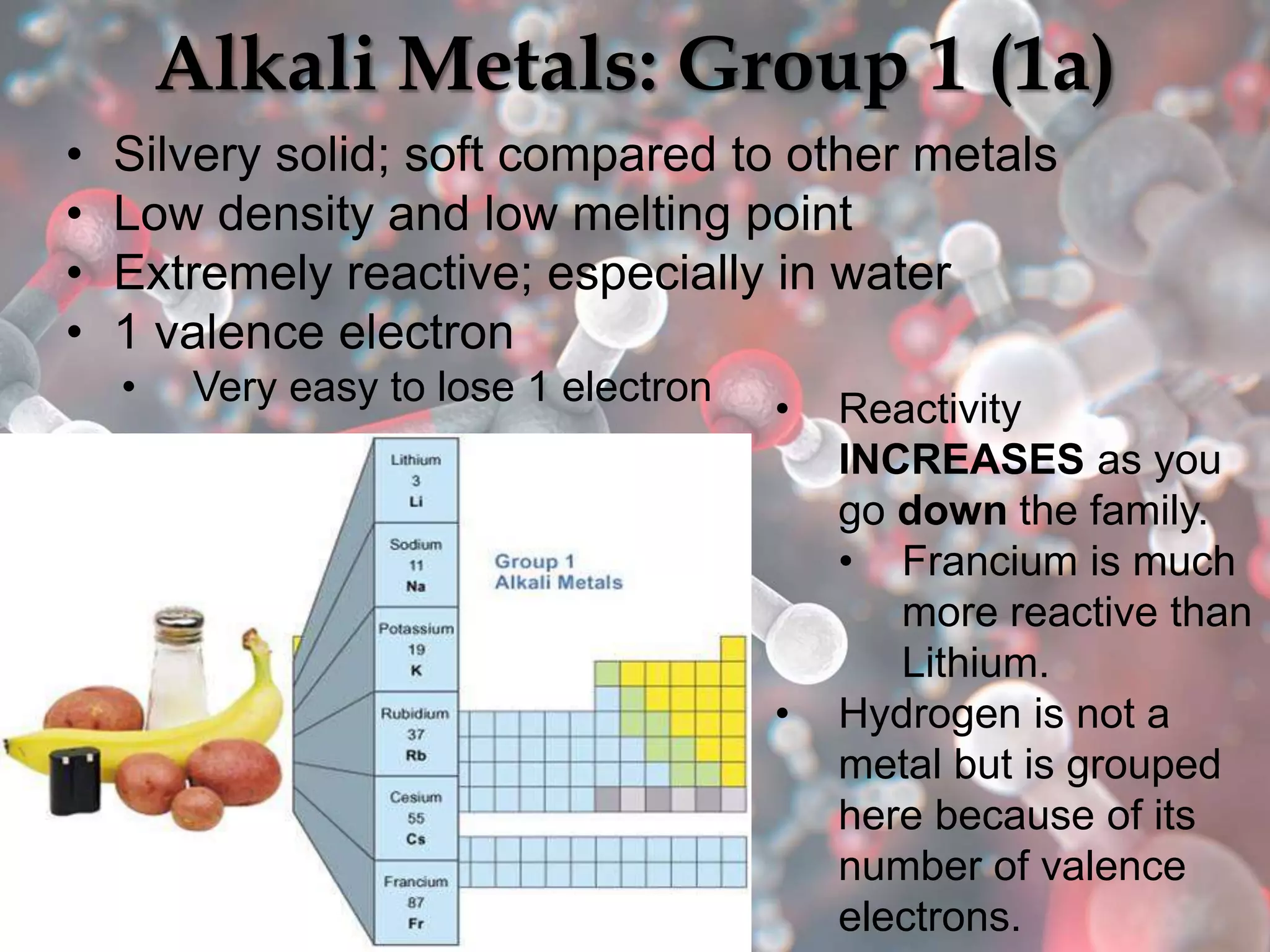
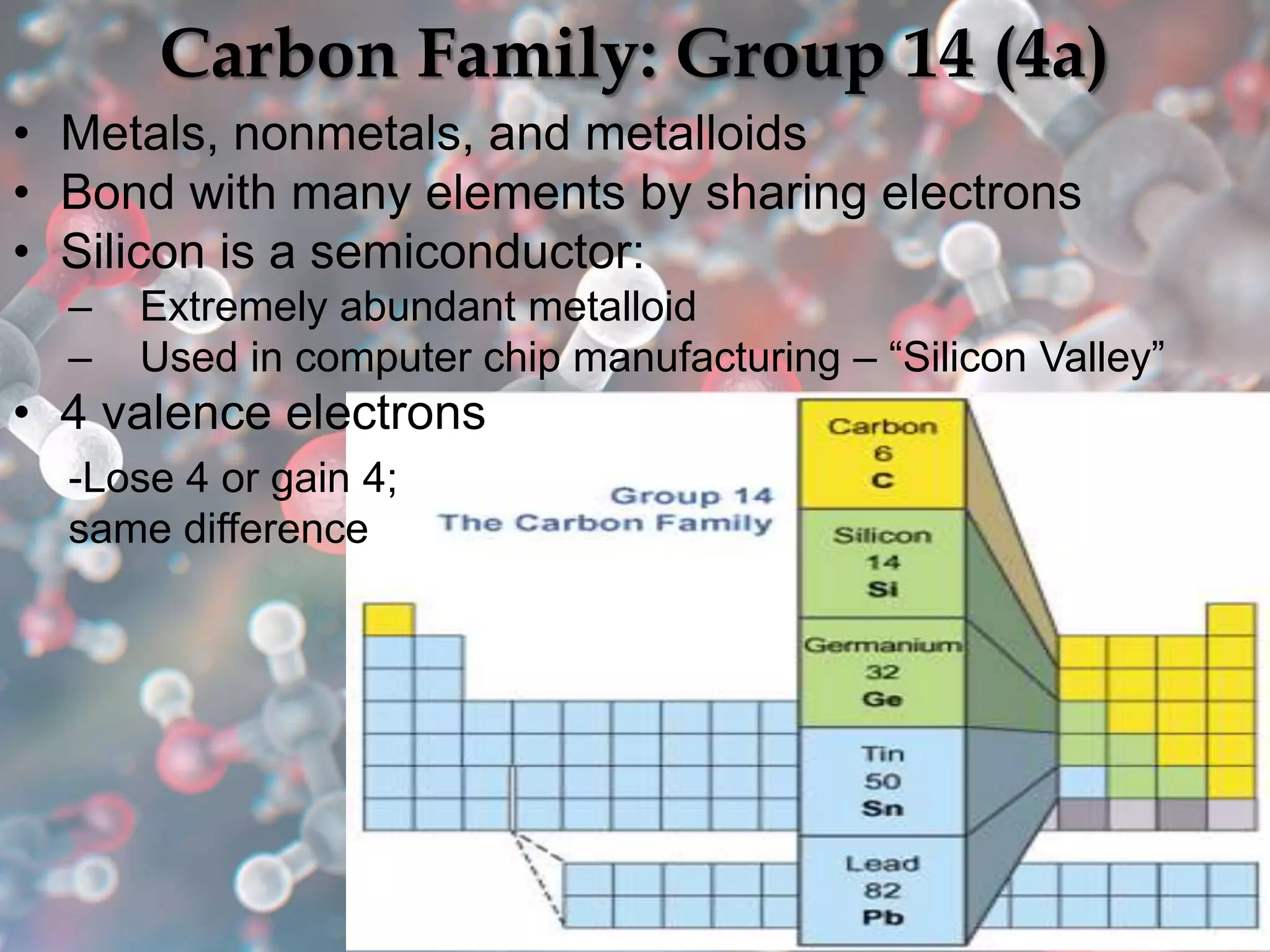
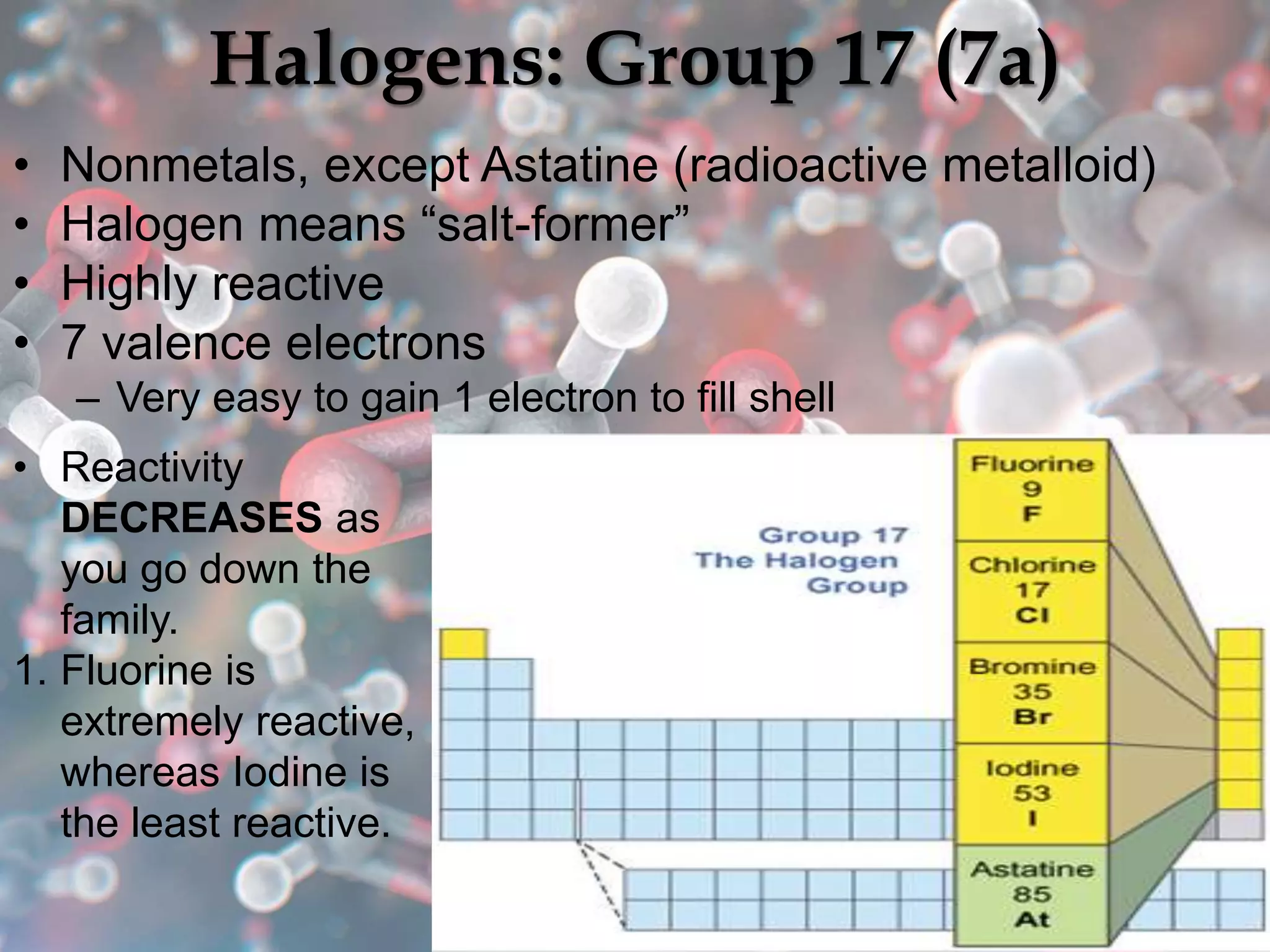
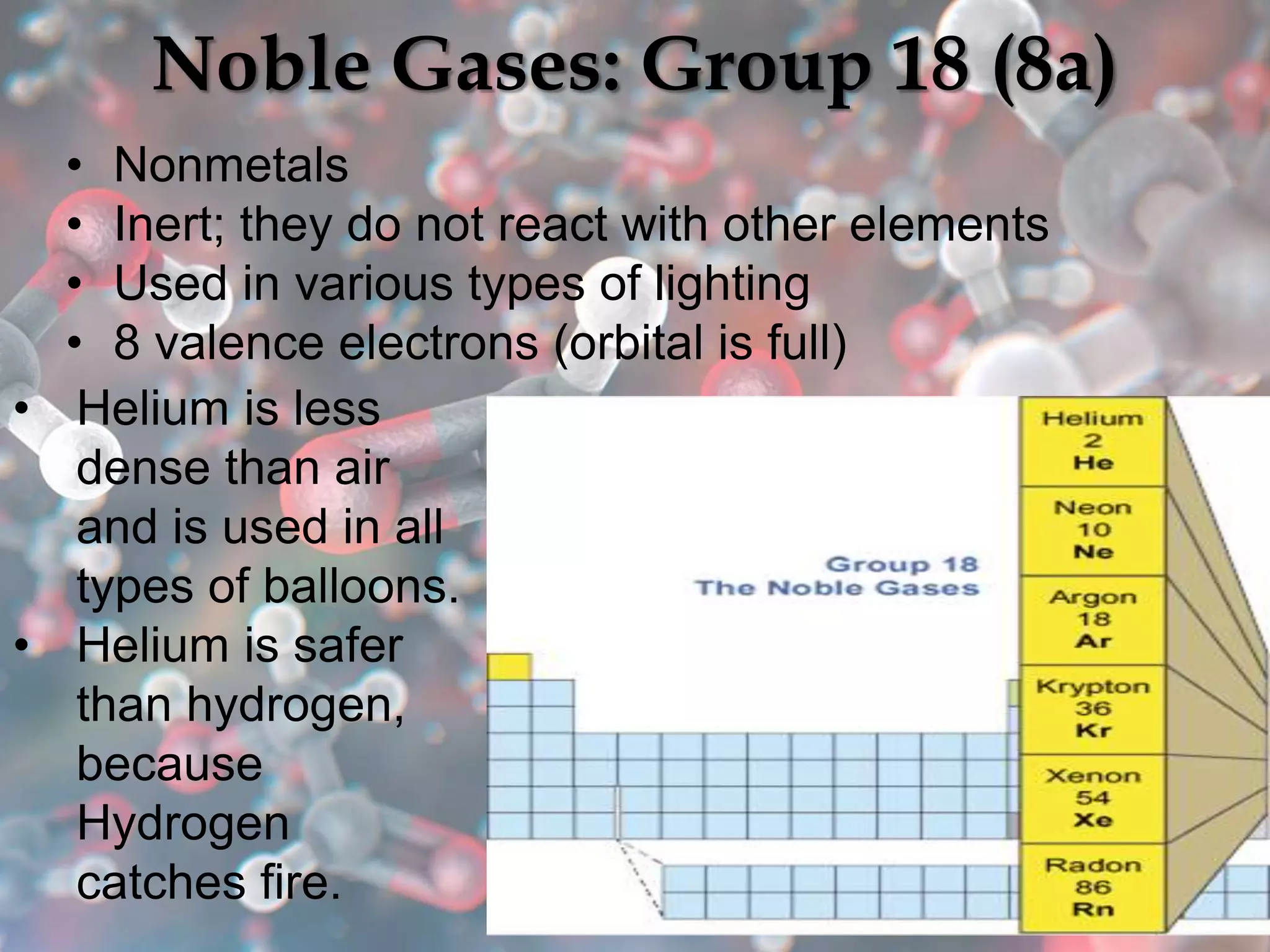
The document provides an overview of the history and development of the periodic table, highlighting key figures such as Johann Dobereiner, John Newlands, Dmitri Mendeleev, and Henry Moseley. It outlines the organization of the modern periodic table, including groups, periods, and classification of elements into metals, nonmetals, and metalloids, as well as various chemical families. Additionally, it discusses properties like reactivity, electron configurations, and the significance of valence electrons in determining chemical behavior.