Embed presentation
Downloaded 91 times


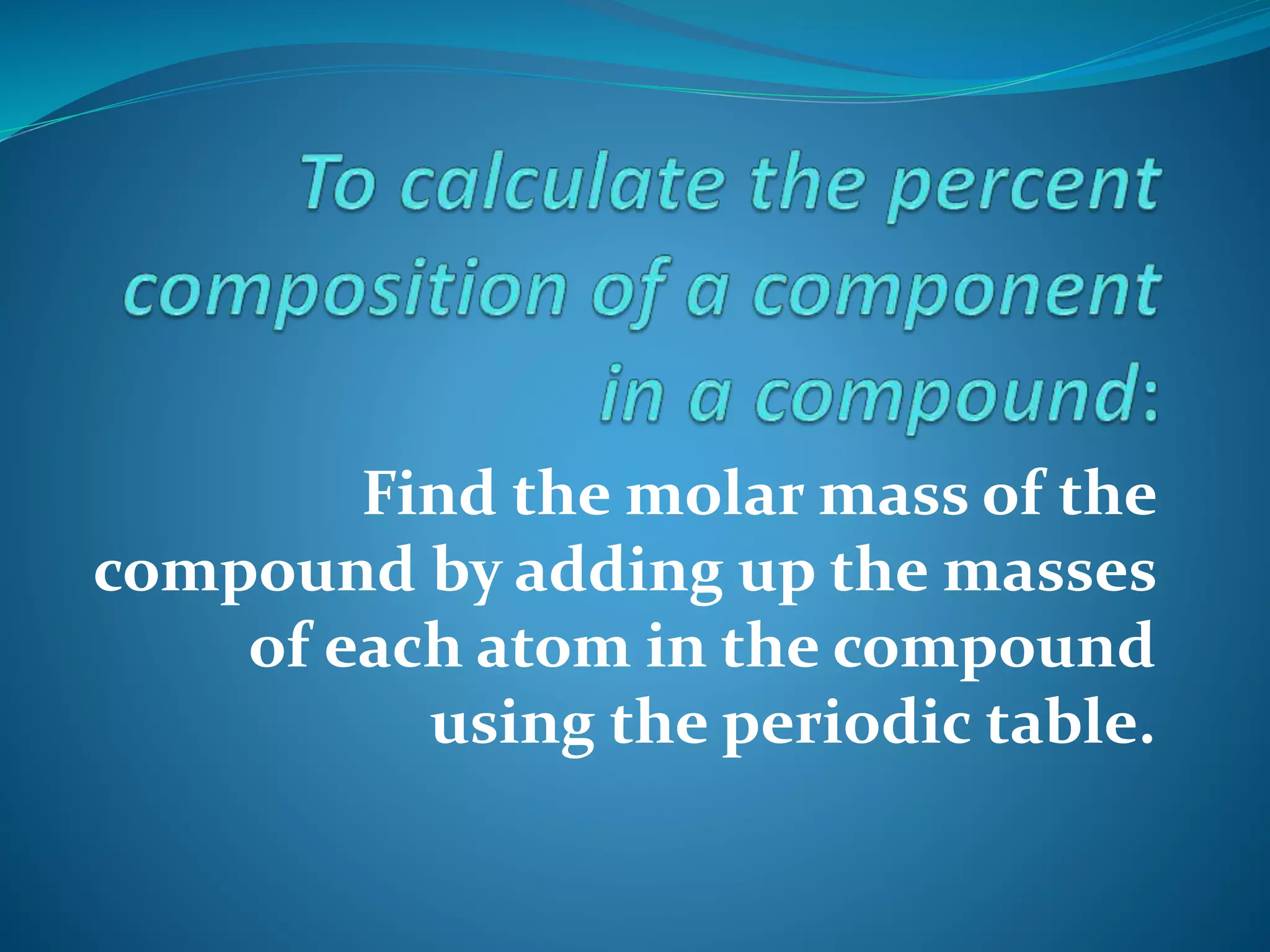




Percent composition is calculated by finding the molar mass of a compound using the periodic table, determining the mass contributed by the component of interest, and dividing that mass by the total molar mass of the compound before multiplying by 100. This allows you to determine the percentage of a compound's total mass made up of a particular component by mass. The document provides an example calculating the percent composition of carbon in carbon dioxide (CO2).





