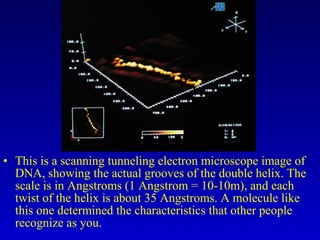
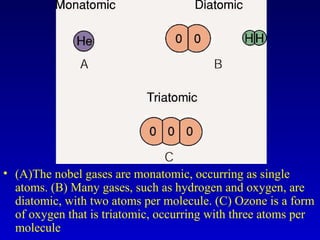
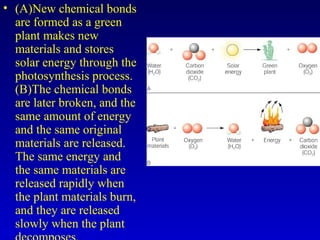
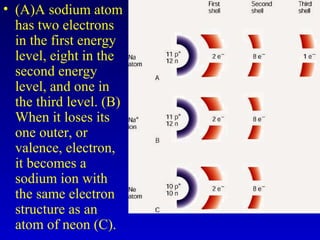
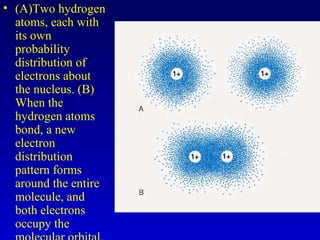
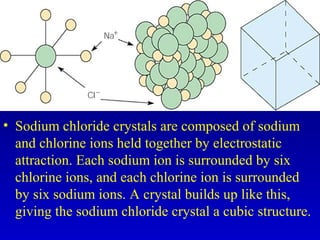
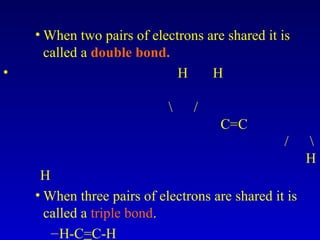
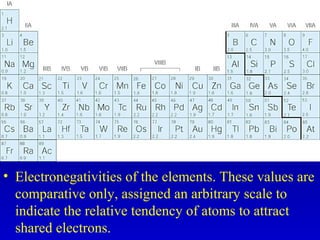
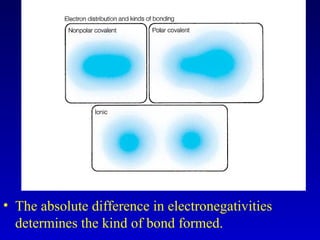
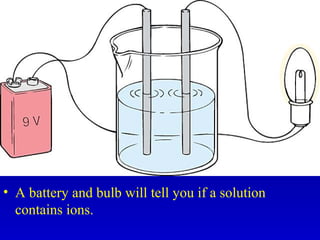
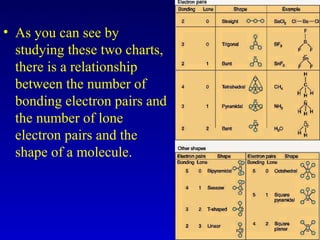
This document provides information about chemical compounds and chemical reactions. It defines key terms like atoms, molecules, chemical bonds and ions. It describes the types of chemical bonds including ionic bonds formed by electron transfer, covalent bonds formed by electron sharing, and polar covalent bonds where bonding electrons are shared unequally. Metallic and coordinate covalent bonds are also discussed. The document explains ion formation and gives examples of naming and writing formulas for ionic and covalent compounds.