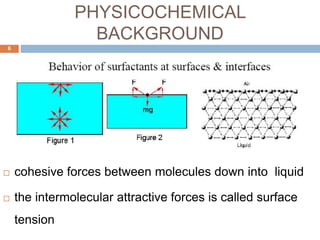
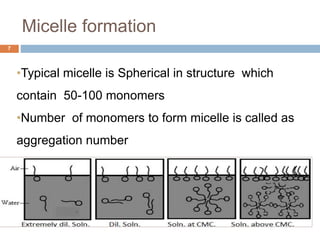
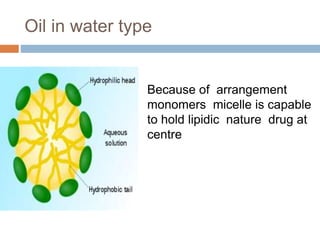
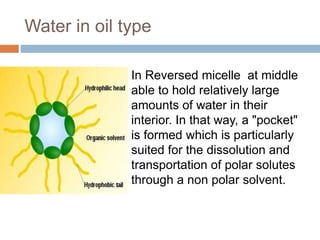
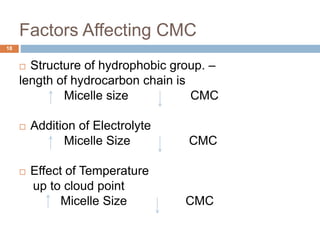
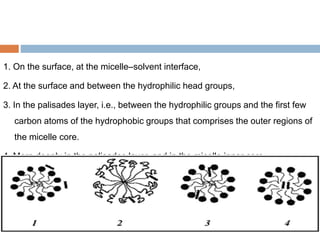
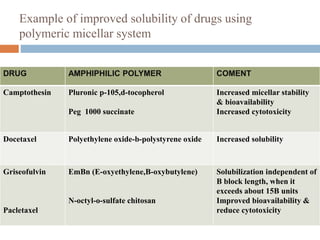
Micellization and their pharmaceutical applications. Micelles are aggregates of surfactant molecules that form spontaneously above a critical concentration. They consist of a hydrophobic core surrounded by a hydrophilic shell. Micelles can increase the solubility of poorly soluble drugs and protect drugs from degradation, thus improving their stability and bioavailability. They also show potential for targeted drug delivery applications such as cancer therapy. Factors like critical micelle concentration, temperature, and electrolyte concentration affect micelle formation and properties.