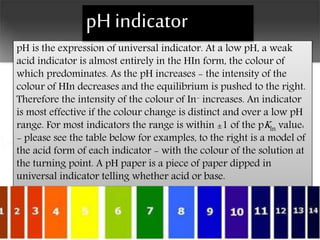
Indicators are chemical substances that change color based on whether a solution is acidic or basic. Common acid-base indicators include litmus, methyl orange, and phenolphthalein. They change color at specific pH levels, allowing for the determination of a solution's acidity or basicity. Other types of indicators include universal indicators, which change color gradually over a wide pH range, olfactory indicators detected by smell, and redox indicators sensitive to oxidation-reduction reactions. Indicators find uses in titrations, testing soils and swimming pools, and monitoring wastes.