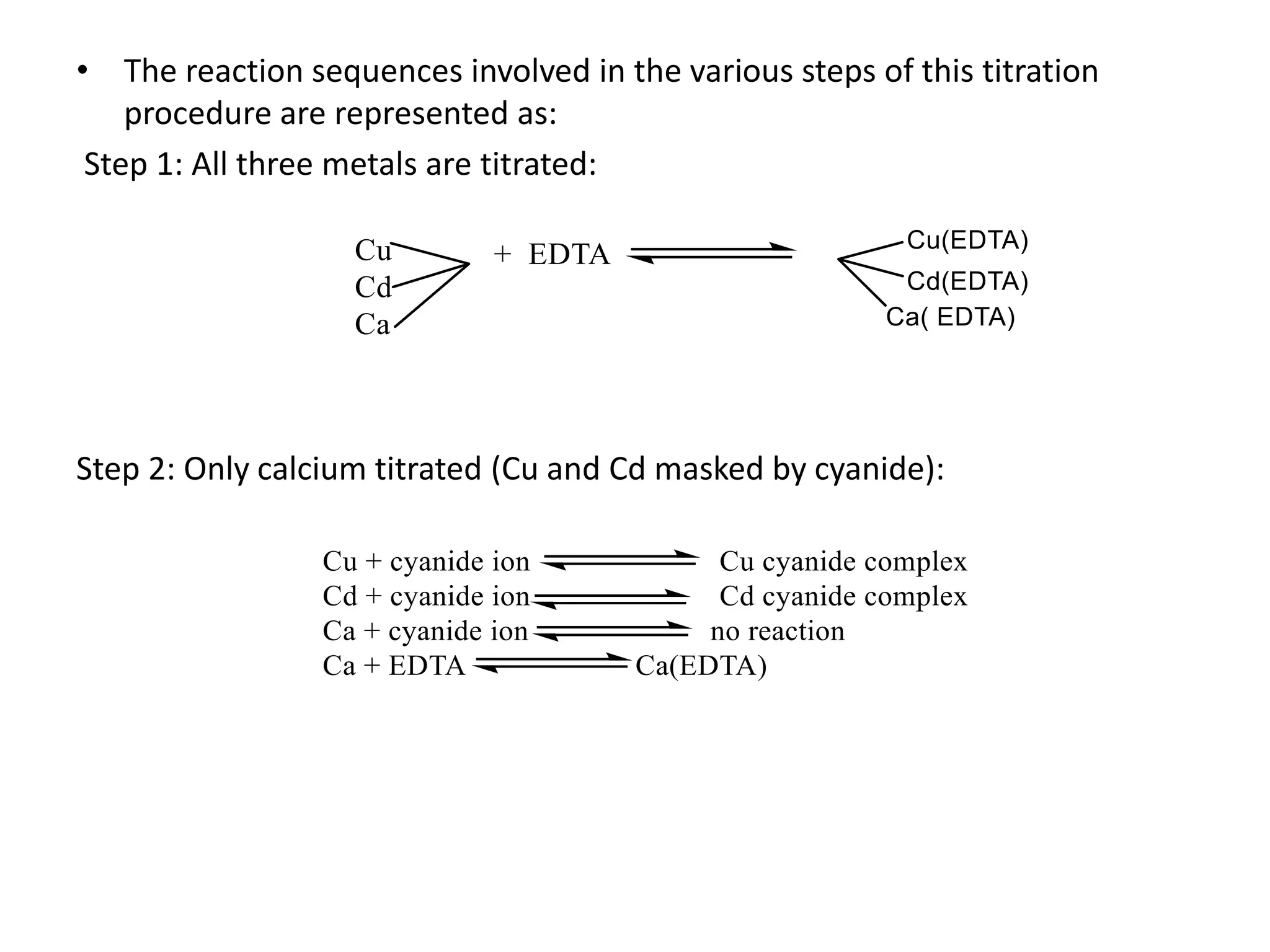
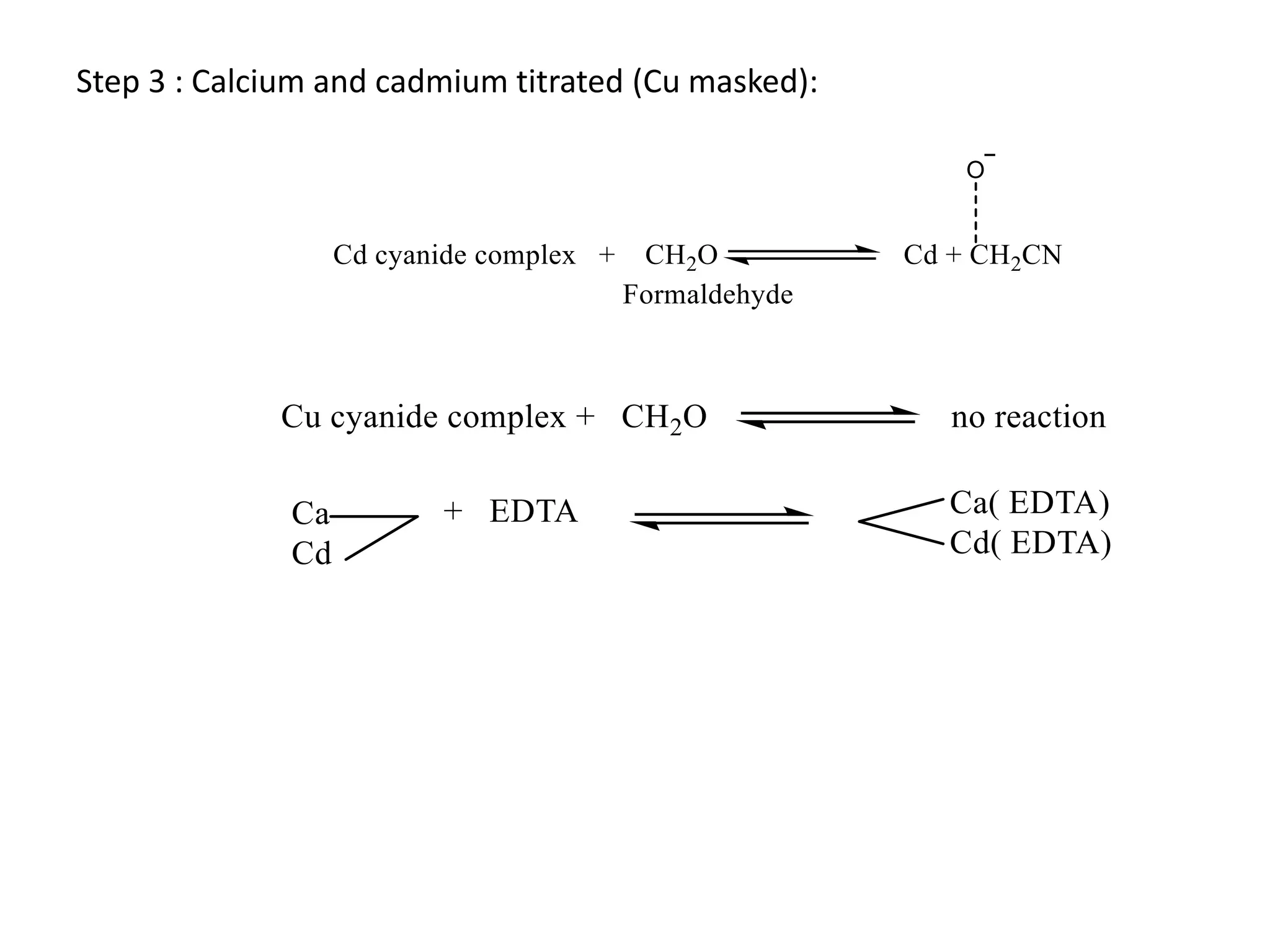
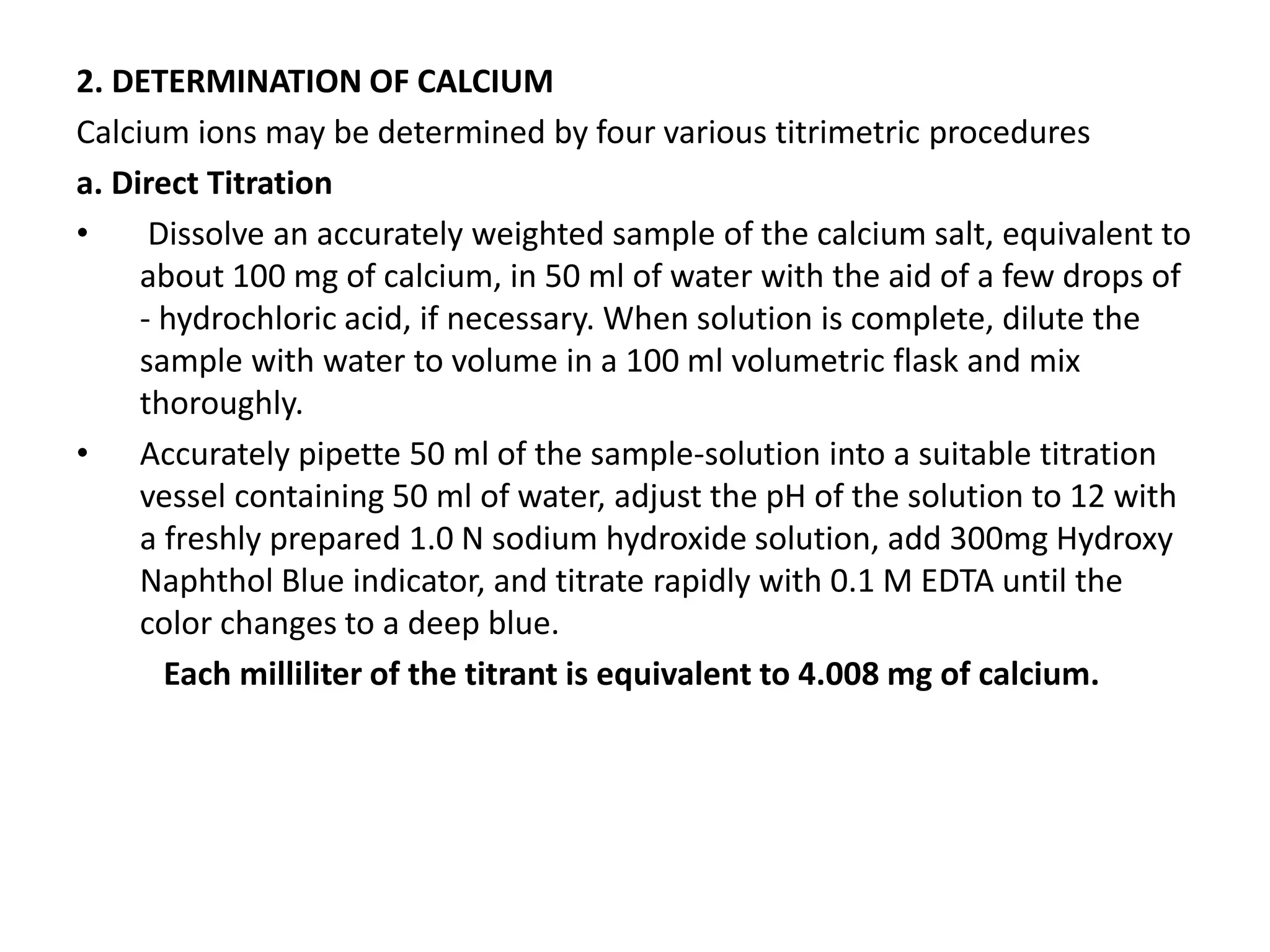
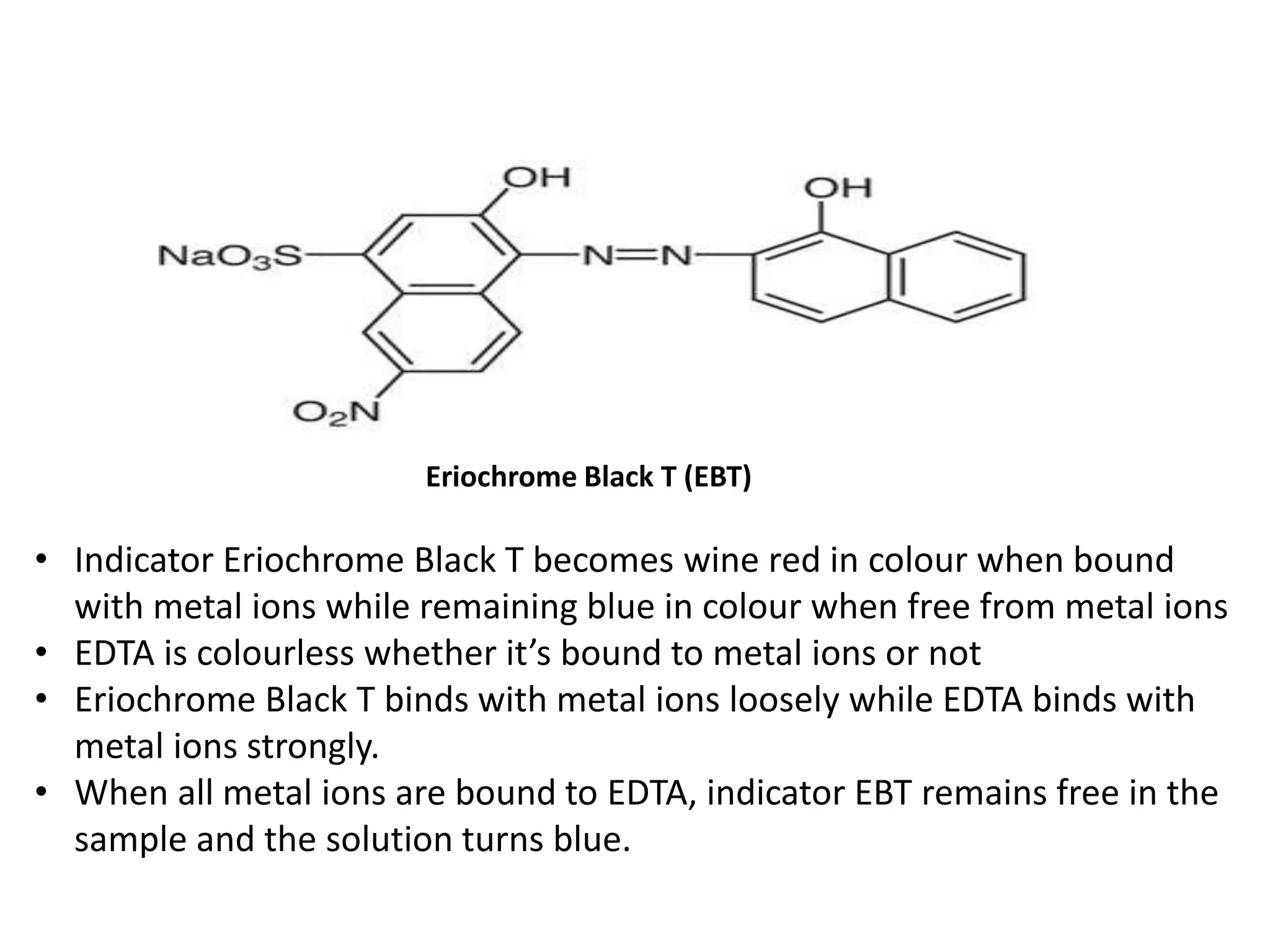
This document discusses complexometric titration, also known as chelatometry. It involves the titration of a metal ion with EDTA (ethylene diamine tetraacetic acid) to form a stable 1:1 metal-EDTA complex. EDTA is a hexadentate ligand that forms very stable complexes with metal ions. The titration is carried out under basic conditions to ensure the metal-EDTA complex forms. Various methods of complexometric titration are described such as direct titration, back titration, and replacement titration. Factors affecting the stability of metal-ligand complexes and requirements for metal ion indicators are also summarized.
![Complexometric Titration
Metal ion (M+) + EDTA (HY) Metal-EDTA complex (MY) + H+
M+n + H2Y-2 MYn-4 + 2H+
Na4-xHxY can be used to represent any species of EDTA, with x designating the
number of acidic protons bonded to the EDTA molecule.
Due to low solubility of EDTA in water, its disodium dihydrate
EDTA salt i.e. Na2H2Y.2H2O is used.
Complex is formed by the reaction of metal ion (Mn+) with either an anion e.g.[Ag(CN)2]- or
neutral molecule e.g.[Ag(NH3)2]+ The metal ion is known as Central metal atom. The anion
or neutral molecule is known as Ligand (L)](https://image.slidesharecdn.com/complexometric-titration-2-220310131052/75/Complexometric-titration-1-2048.jpg)
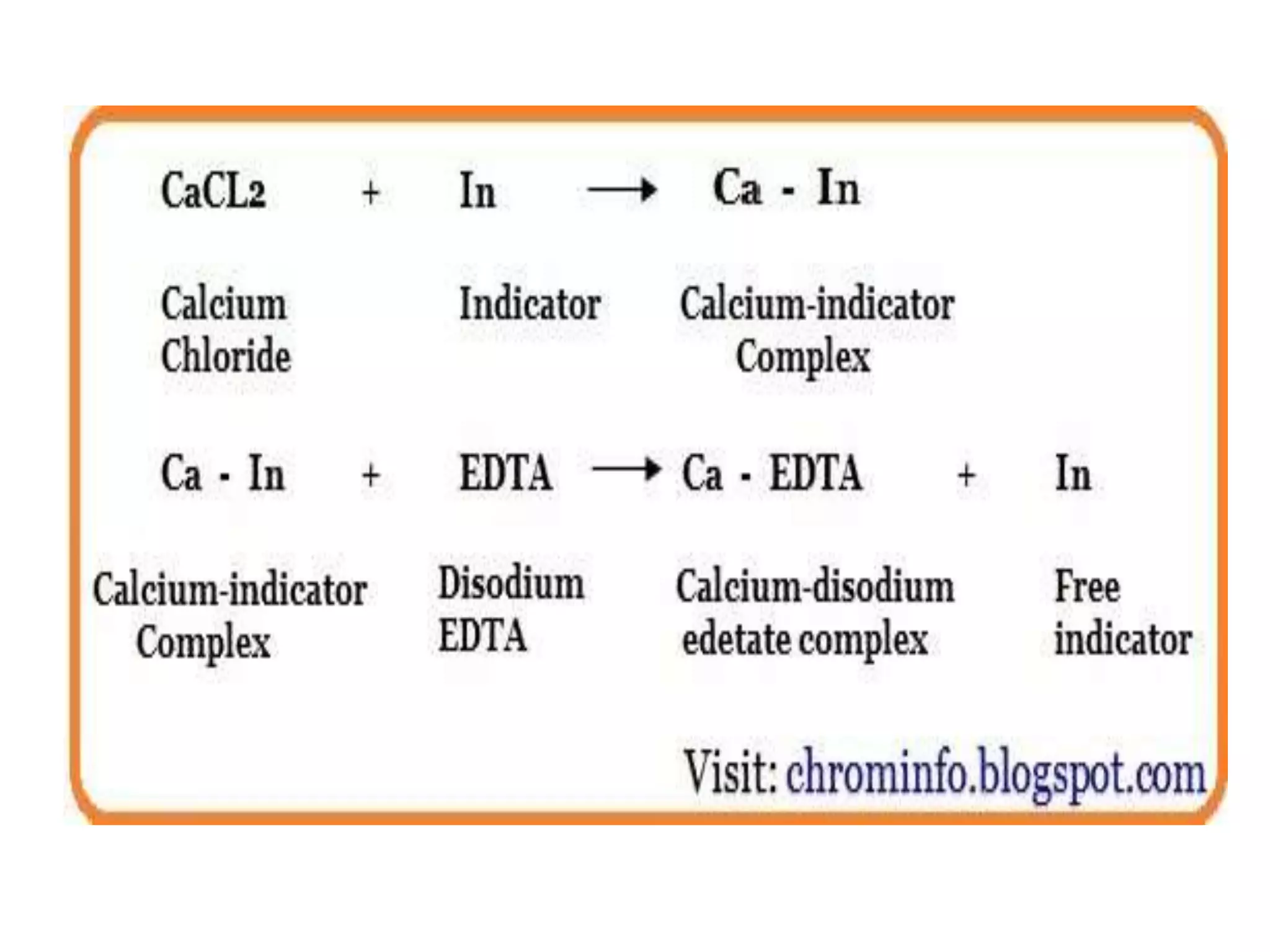
![• It is a volumetric analysis in which formation of a colored complex is used to indicate the
end-point of the titration.
• It is used for the determination of a mixture of different metal ions present in the solution.
• Complexometric titrationChelatometry is based on essentially Lewis acid-base reactions
in which electron pair is donated from one chemical to another.
• Disodium EDTA (Na2H2Y ⋅ 2H2O: better soluble in water) is most common chelating
agent used for Complexometric titration because it creates water soluble stable metal
complex in alkaline medium. (except the alkali metals which form too weak complexes)
• All the complexes have exact 1:1 stoichiometry (regardless of the charge of the cation).
They react stoichiometrically and can be used to quantitatively determine the metal ions
in the sample by titration.
• The titration needs to be carried out at alkaline medium to consume H+ which produced
during reaction, so that the reaction goes to the right and stability of the complex formed
(MY) is increased.
• The versatility, sensitivity, and general convenience of complexometric titrations are
dependent on the correct choice of indicators for endpoint detection.
• In deprotonated form of EDTA , six binding sites—four negatively charged carboxylate
groups and two tertiary amino groups—that can donate six pairs of electrons to a metal
ion to form coordinate covalent bond. [EDTA =Hexadentate ligand]
Some terms
• Ligand: Compound having at least one pair of unshared electrons available for bond formation
• Chelate: It is a complex formed between the ligand containing two or more donor groups and metal
to form ring structure. (heterocyclic rings or chelate rings). Chelates are usually insoluble in water
but soluble in organic solvent.
• Chelating agents: Organic molecules/ligands containing two or more donor groups which combine
with metal to form complex having ring structure. Most chelating agents consist of N or O.
• Sequestering agent: Ligands which form water soluble chelates e.g. EDTA.](https://image.slidesharecdn.com/complexometric-titration-2-220310131052/75/Complexometric-titration-3-2048.jpg)

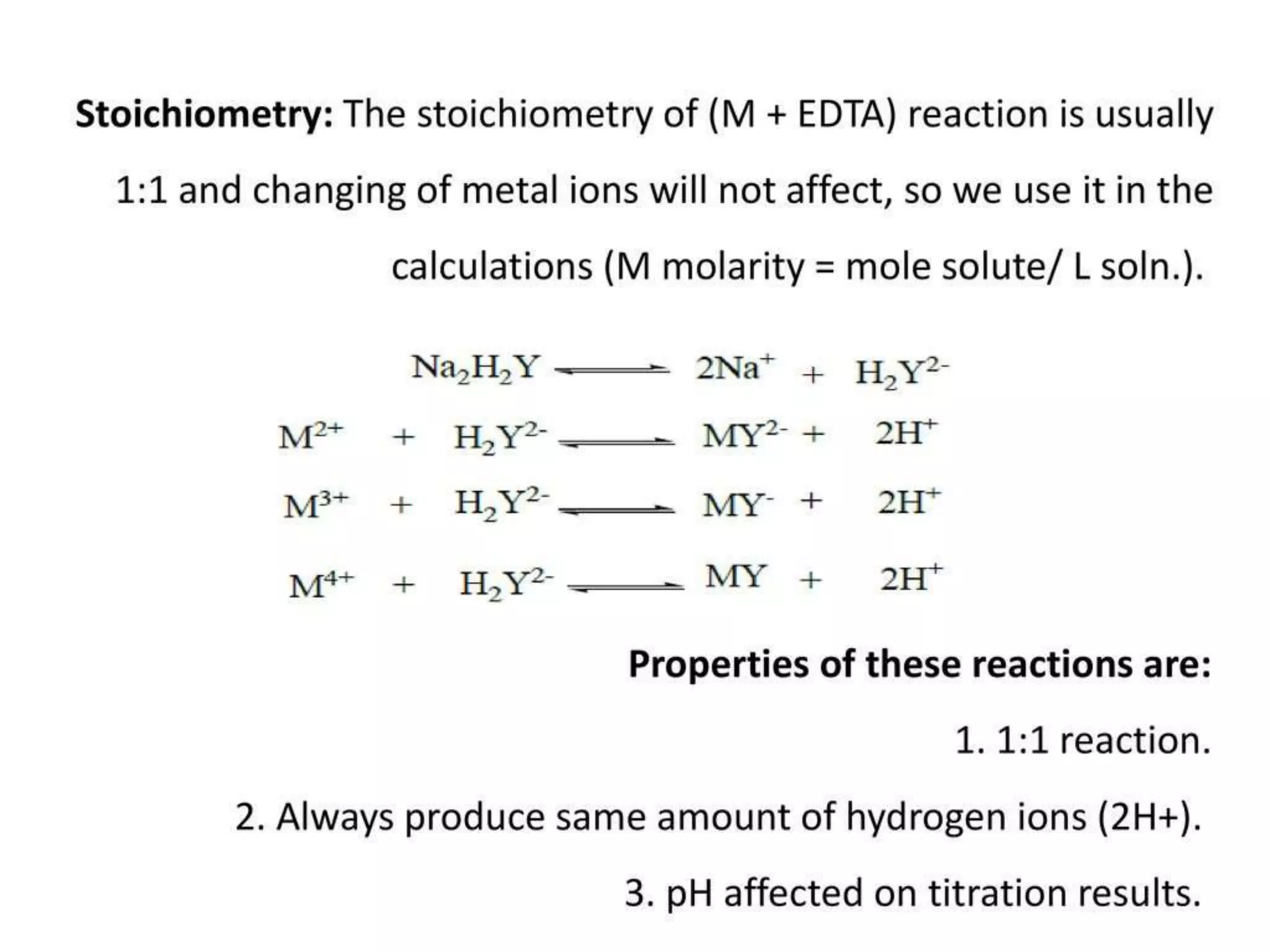


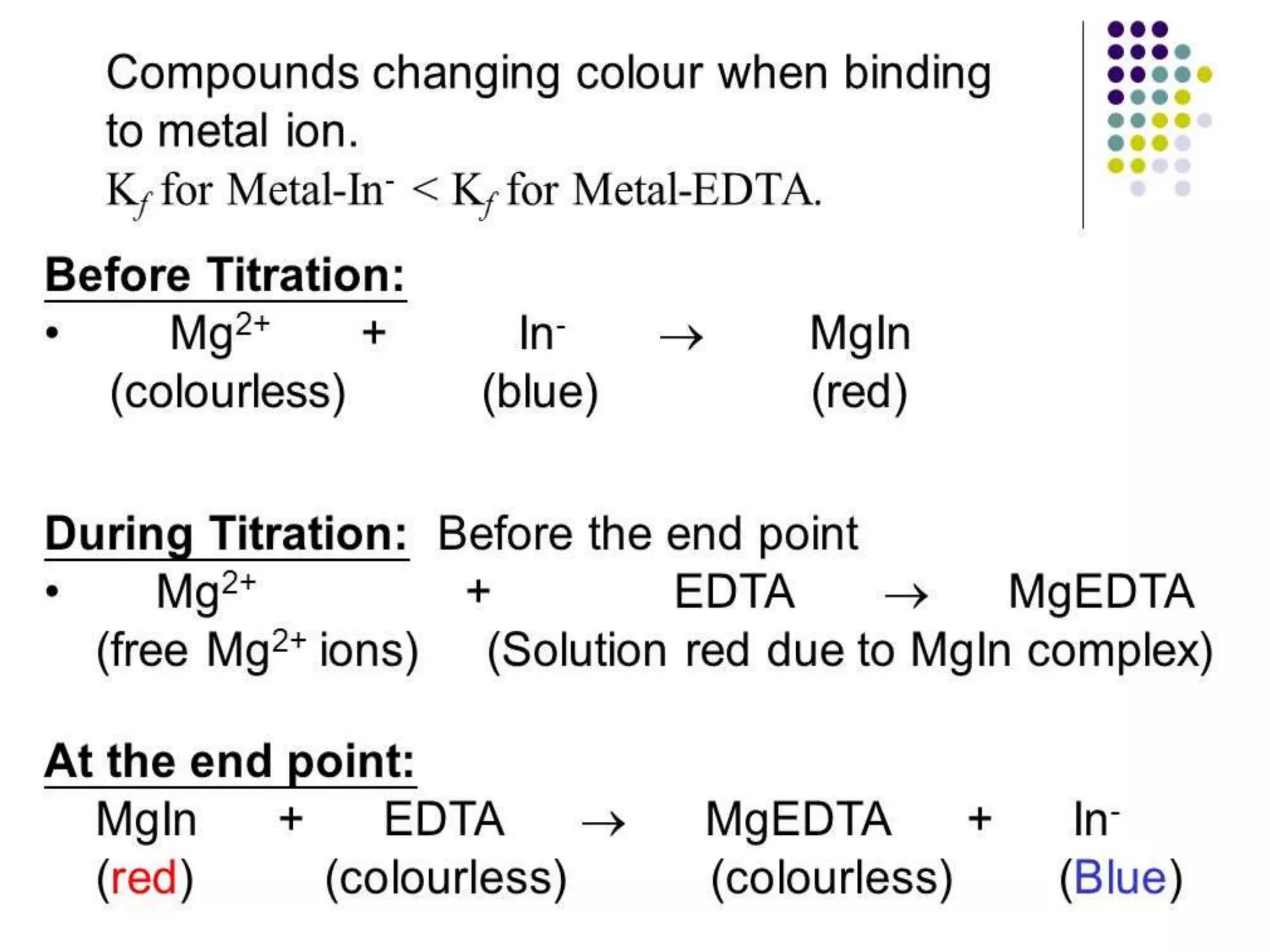
![Effect of pH on complex formation:
• During the formation of stable, 1:1 water soluble, colorless complex there
will be the release of two H+ ions, as the number (n) of M-EDTA complex
increases in the solution, there will be the removal of 2n[H+] ions, acidity
increases in the solution. So we need to maintain the pH of the solution
with suitable basic buffer in order to have a M-EDTA complex. Hence
complexometric titrations are generally carried out in basic pH.
• Therefore, the stability of the metal complex is pH dependent. The lower
the pH of the solution the more hydrogen ions are available to compete
with the metal ion for the ligand, and the equilibrium shifts to the left,
causing a decrease in the stability of the complex. This equation
illustrates the competition between the metal ion and the hydrogen ion
for the ligand: M2+ + H2Y2- = MY2- + 2H+](https://image.slidesharecdn.com/complexometric-titration-2-220310131052/75/Complexometric-titration-8-2048.jpg)
![STABILITY OF COMPLEXES
• Generally, the formation of a 1 : 1 chelate complex (MY n − 4 )may be
designated by the following equation :
where, M = Metal ion, and X = Chelating ion.
Hence, the stability constant, Kf , may be expressed as :
Kf =[MYn-4]/[Mn+][Y4-]
The equilibrium constant for the reaction of a metal with a ligand is called the
formation constant, Kf , or the stability constant.
The formation constant Kf = [MY n − 4 ] /[Mn+][Y4− ] describes the reaction between
Y4− and a metal ion.
Generally, complex ions with polydentate ligands have much higher formation
constants than those with monodentate ligands. This additional stability is
known as the chelation effect](https://image.slidesharecdn.com/complexometric-titration-2-220310131052/75/Complexometric-titration-9-2048.jpg)









![• The potential of the electrode is given by Nernst equation: When the
potential of the system is measured, the standard potential E0 is known,
and the concentration of free mercury(II) ion is calculated by:
• E= E0-(0.0591/2) log[Hg2+ ]
• In the titration procedure using mercury electrode system, the electrode is
immersed in the solution containing the metal ion to be titrated and a
small quantity of a mercury(II)-EDTA chetate (usually a few drops of a 0.01
M solution),](https://image.slidesharecdn.com/complexometric-titration-2-220310131052/75/Complexometric-titration-27-2048.jpg)
![• Mercury(II) is displaced from the mercury(II)-EDTA chelate by the metal ion
(that is, a divalent ion M2+ ), and the following exchange equilibrium results:
• From this equilibrium it can be seen that the concentration of M2+ , which is
in excess of the Hg-EDTA2- at the start of the titration, determines the
amount of free mercury(II) in solution ;thus the potential of the mercury
electrode depends upon the ratio [M2+ ]/[M-EDTA 2-].
• During the early part of the titration with EDTA, the ratio [M 2+ ]/[M-EDTA2-]
changes only slightly; however, in the vicinity of the end point this ratio
undergoes its largest changes and the potential of the mercury electrode
changes accordingly. As in all potentiometric titrations, the equivalence
point is indicated by a sharp inflection in the titration curve.](https://image.slidesharecdn.com/complexometric-titration-2-220310131052/75/Complexometric-titration-28-2048.jpg)