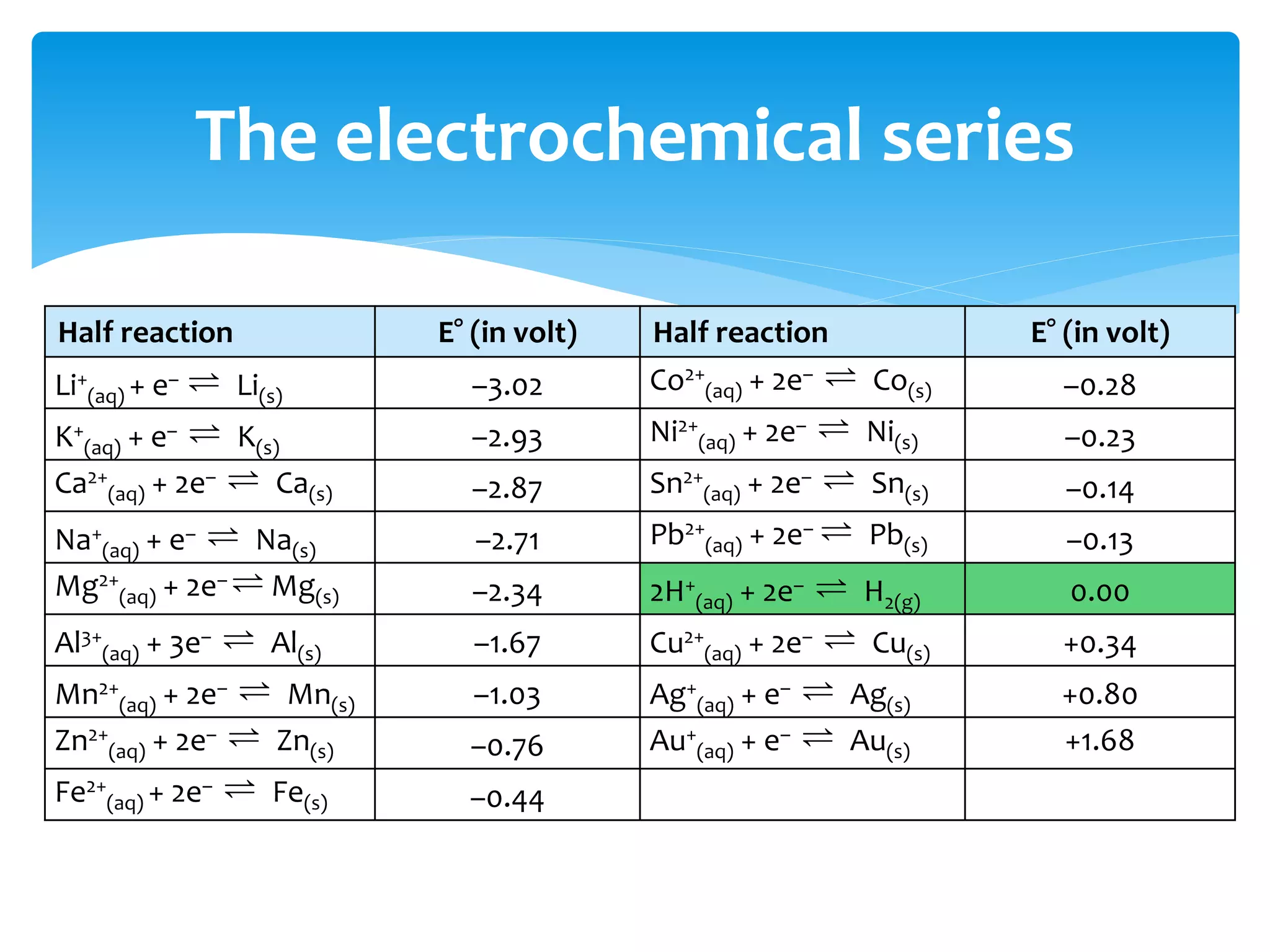
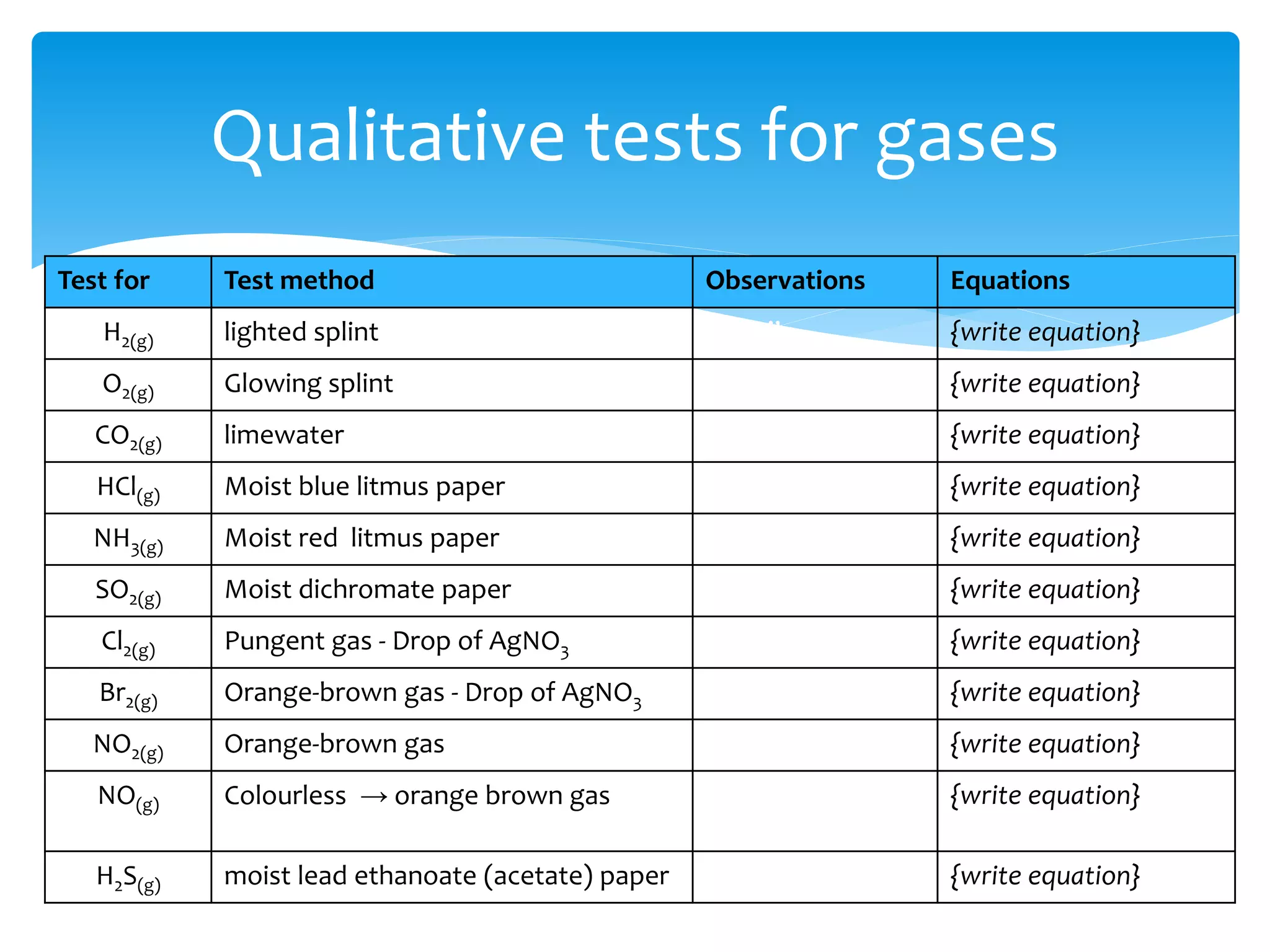
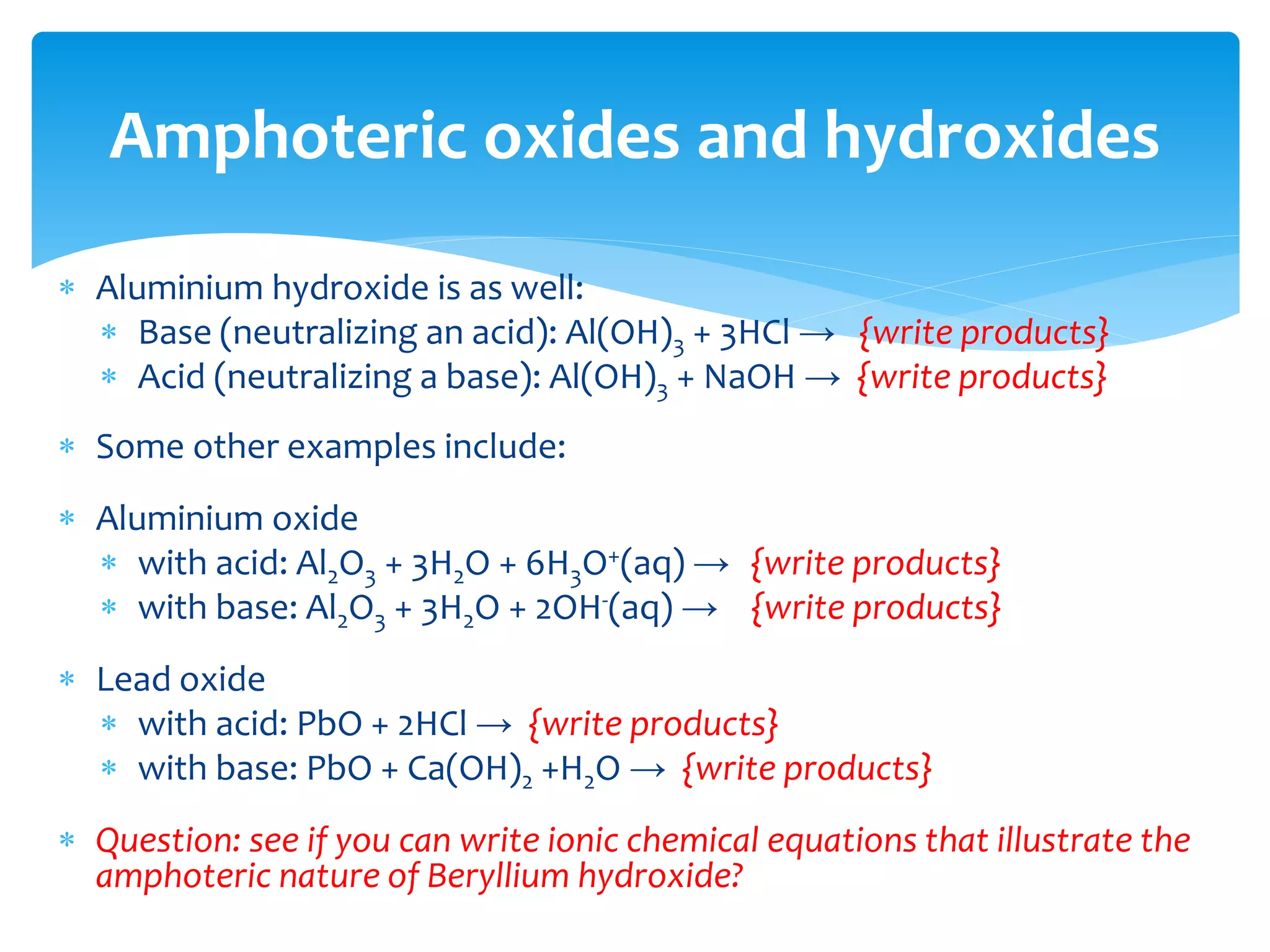
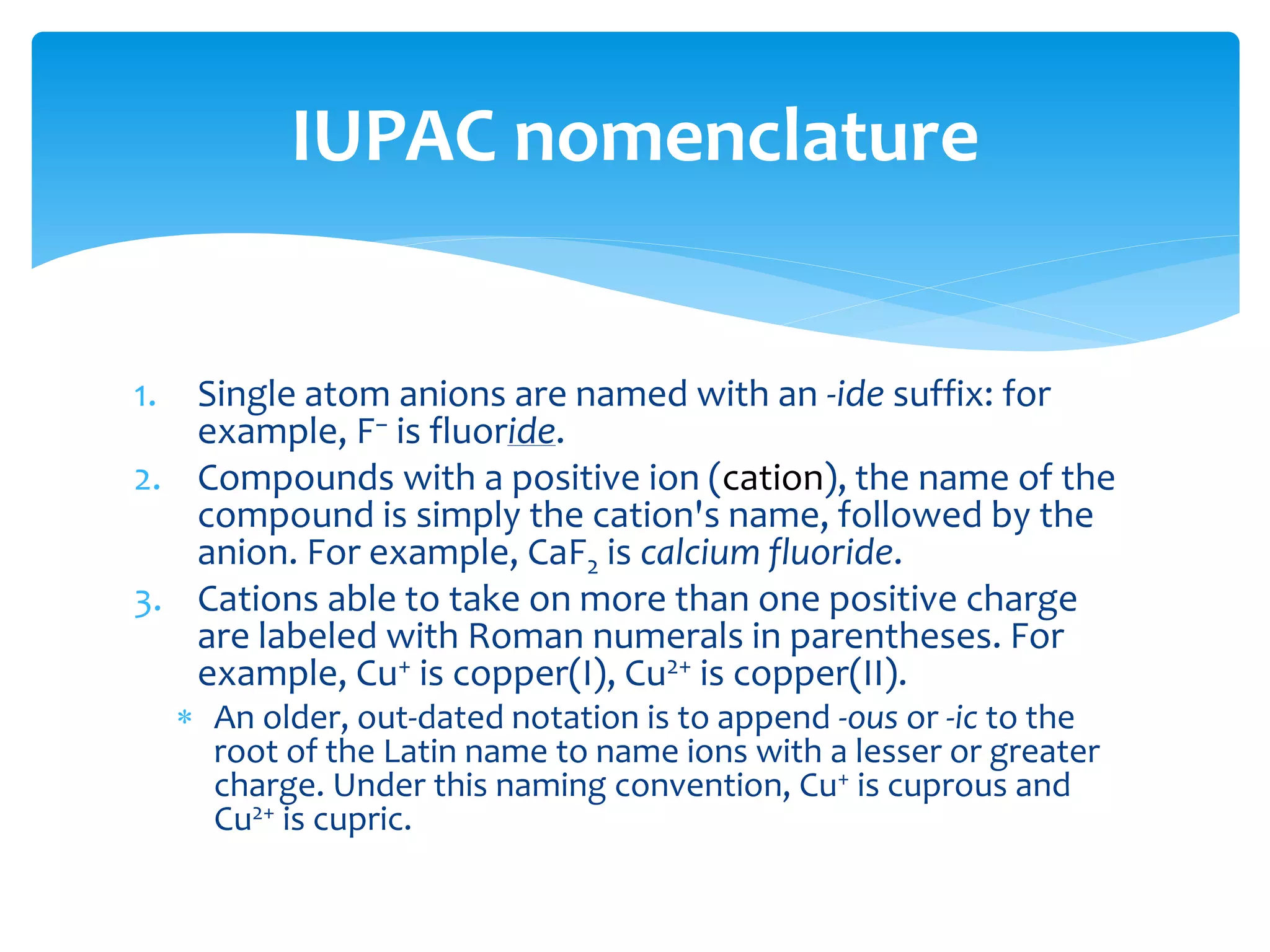
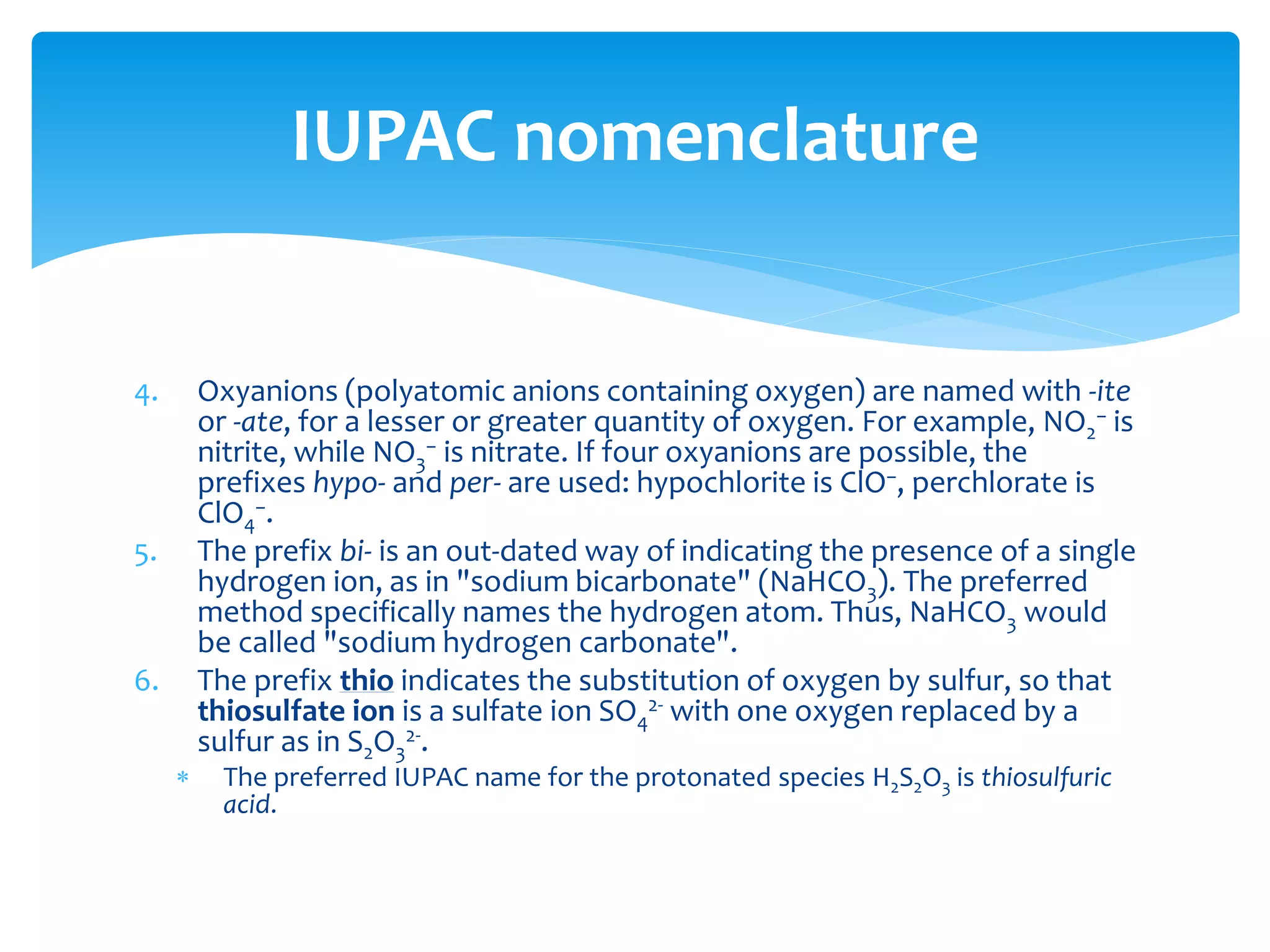
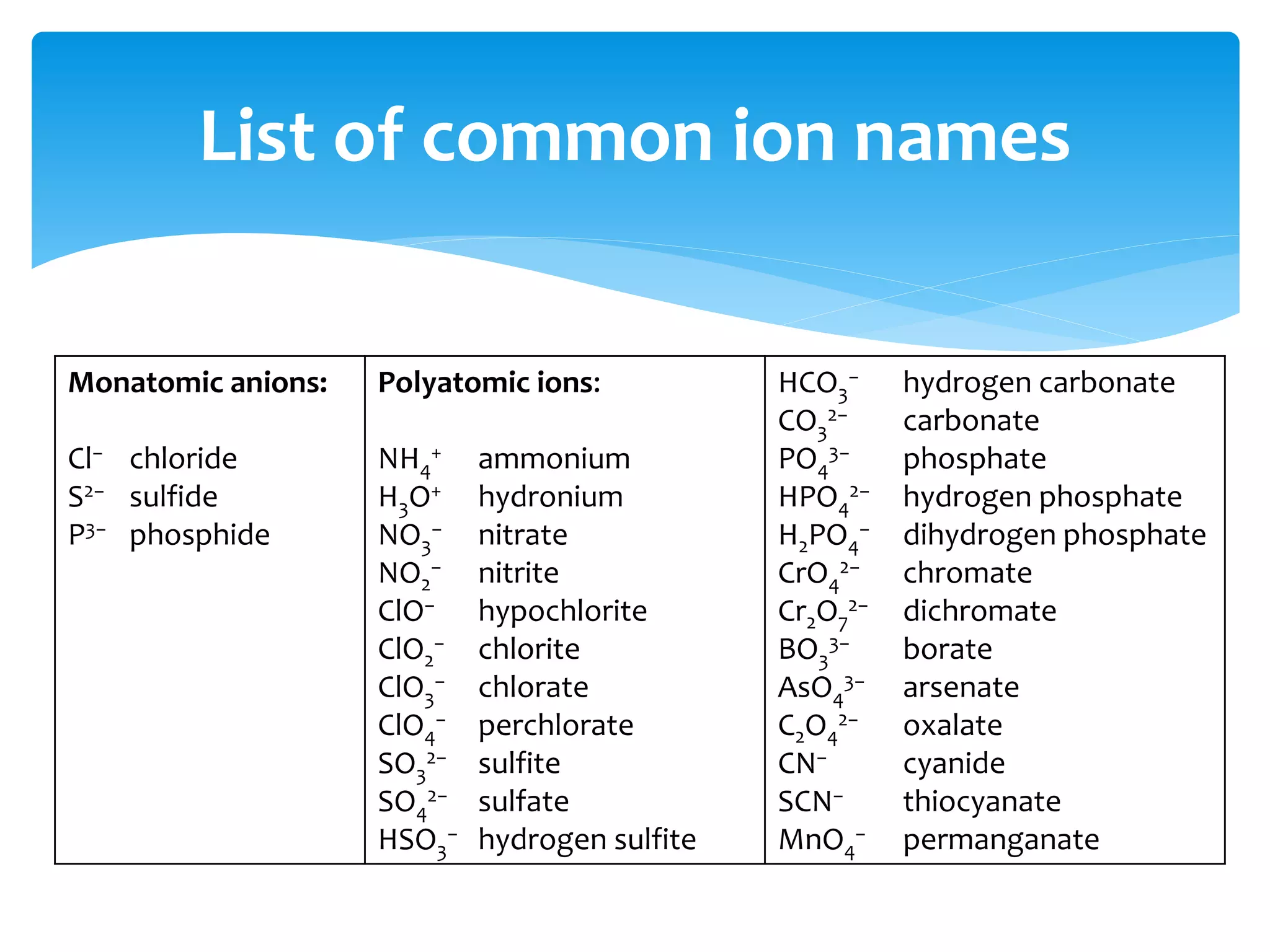
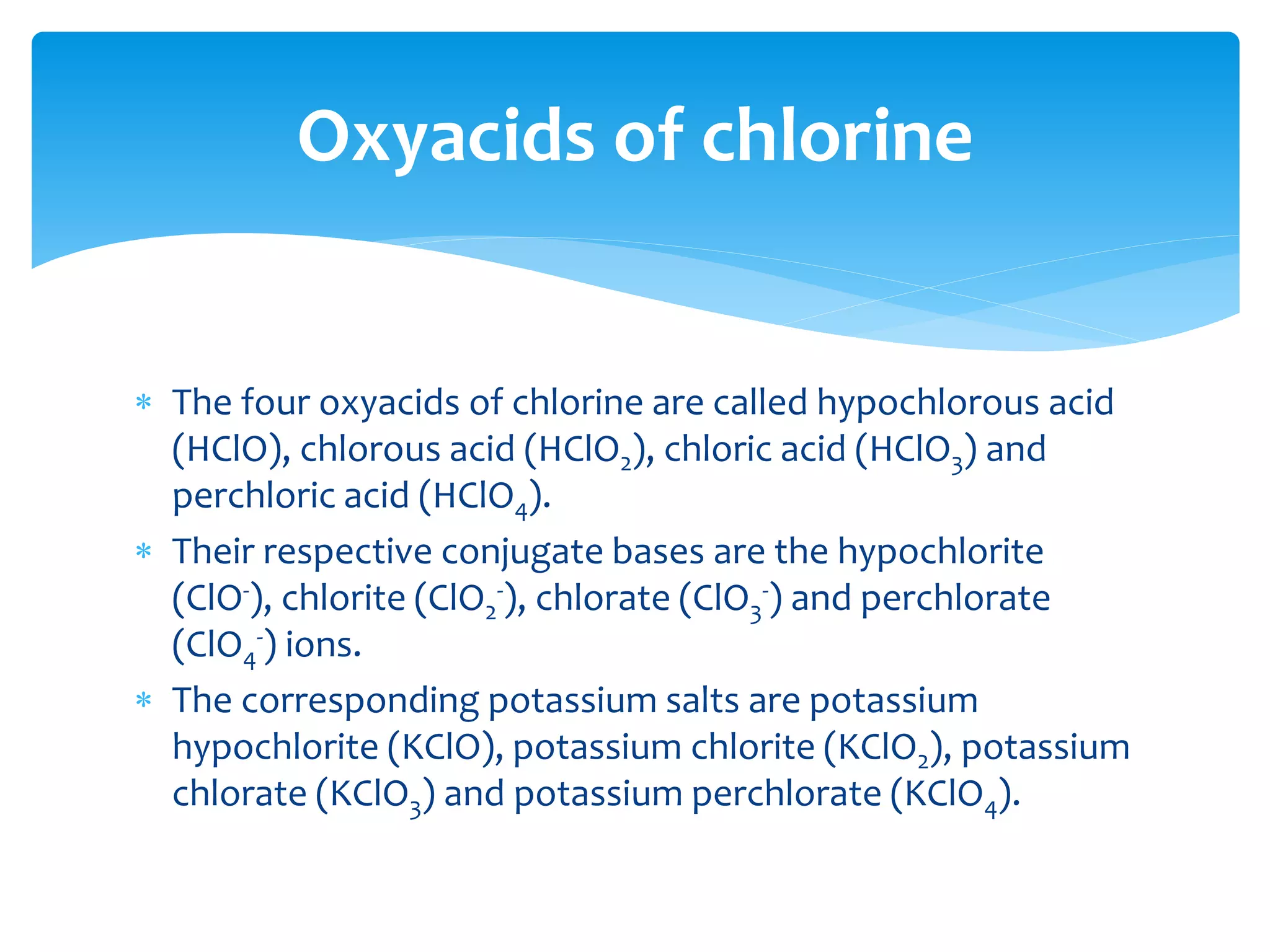
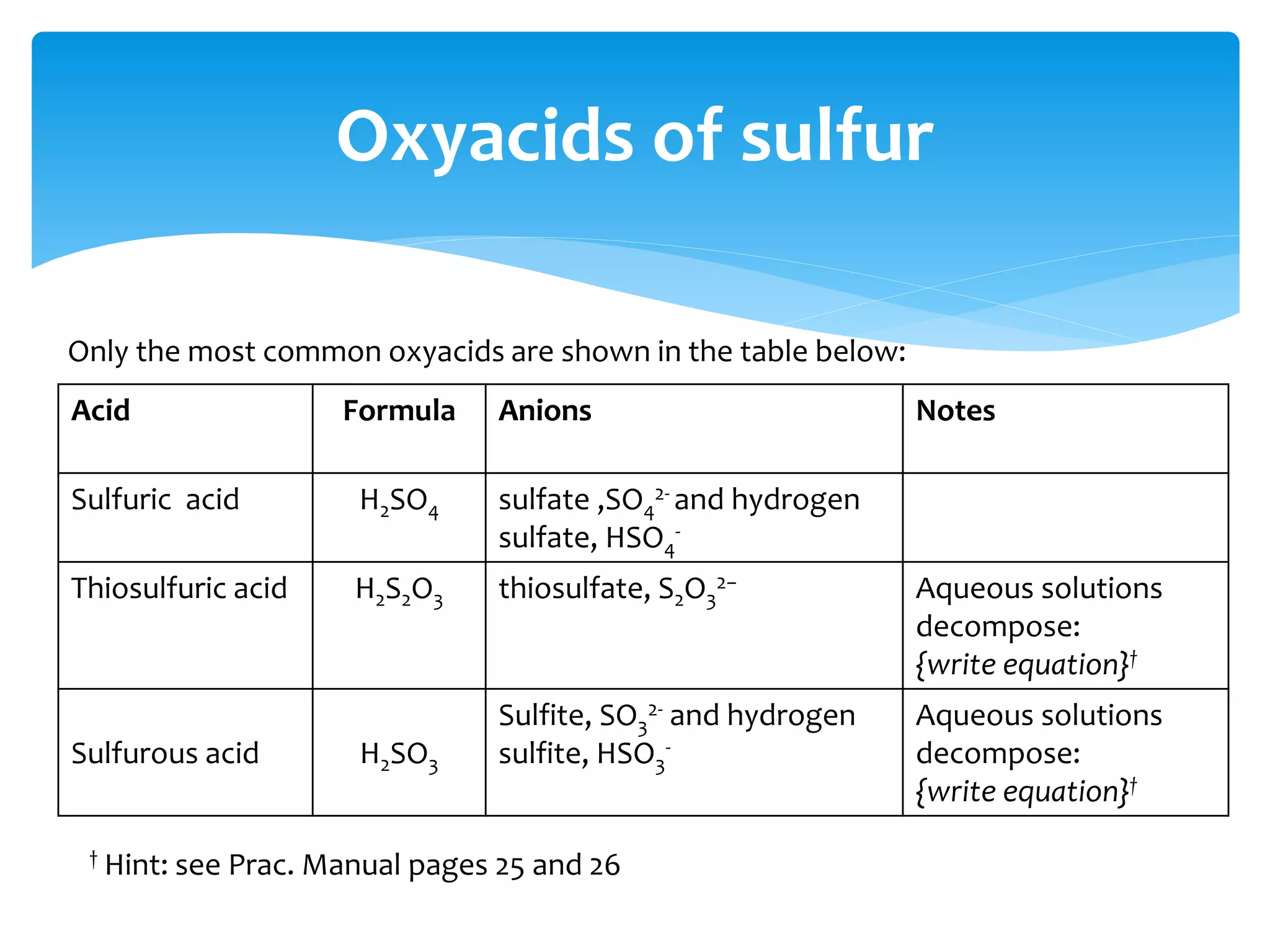
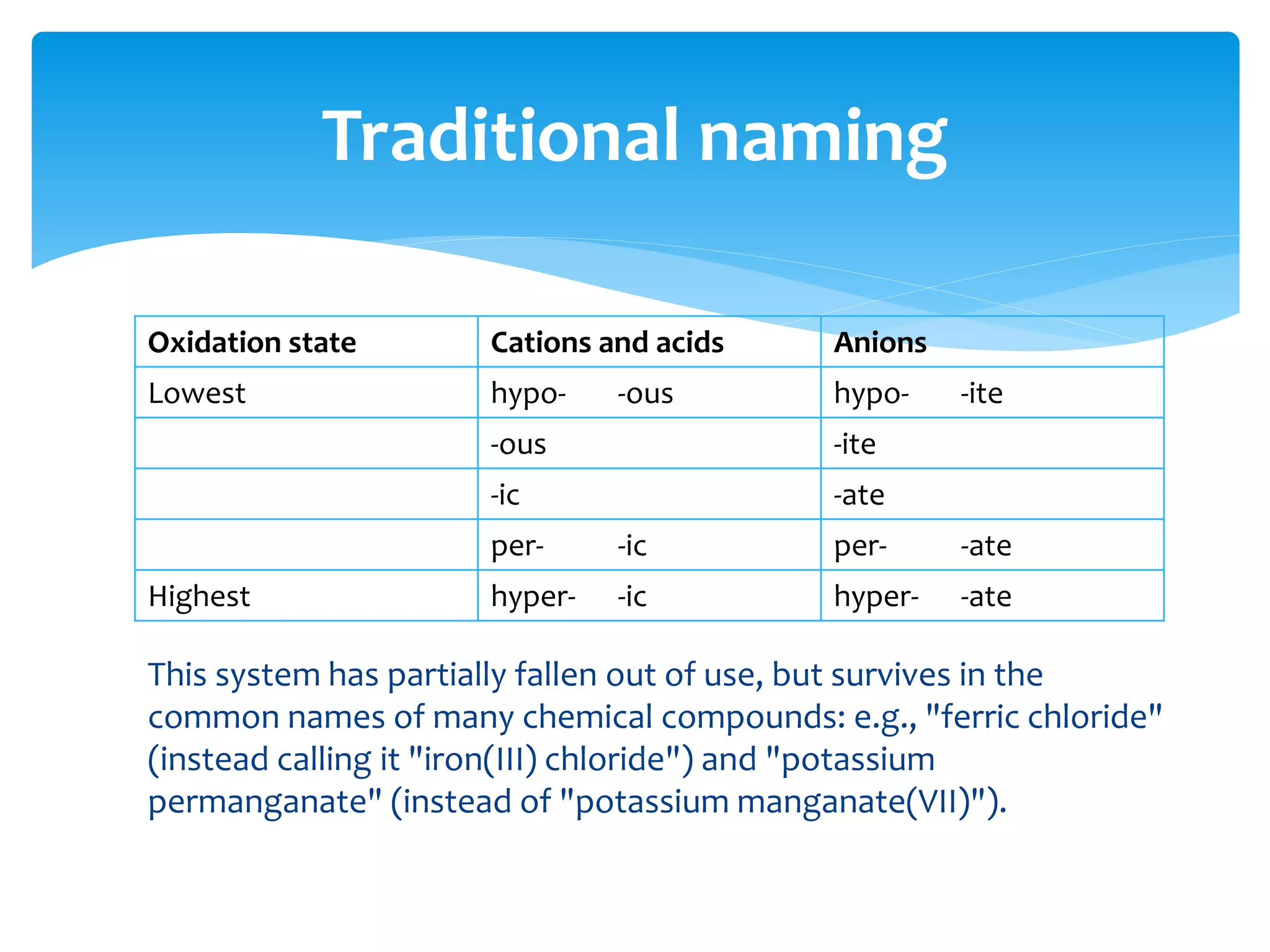
The document outlines the curriculum and objectives for a qualitative analytical chemistry course at QUT, emphasizing the importance of chemical literacy and practical laboratory skills. It offers guidance on report writing, chemical nomenclature, and safe handling practices for acids and bases. Additionally, it includes information on various chemical compounds, their properties, and proper procedures for lab safety.













![ Write the name of the central atom/ion. If the complex is an
anion, the central atom's name will end in -ate, and its Latin
name will be used if available (except for mercury).
If the central atom's oxidation state needs to be specified, write
it as a Roman numeral in parentheses.
Name cation then anion as separate words.
Examples:
[NiCl4]2− tetrachloridonickelate(II) ion
[CuNH3Cl5]3− amminepentachloridocuprate(II) ion
[Cd(en)2(CN)2] dicyanidobis(ethylenediamine)cadmium(II)
[Co(NH3)5Cl]SO4 pentaamminechloridocobalt(III) sulfate
Naming complexes](https://image.slidesharecdn.com/qualitativeanalysis1-150219211511-conversion-gate01/75/Qualitative-analysis-1-21-2048.jpg)