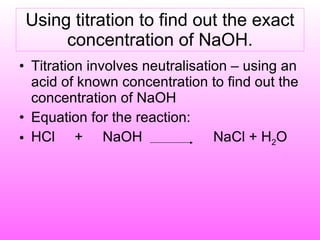
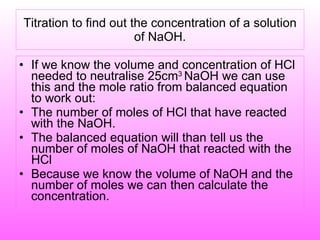
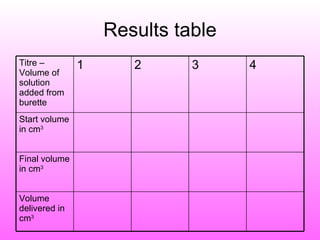
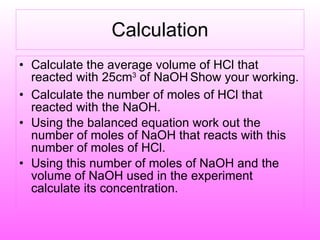
The document discusses volumetric analysis techniques for determining the concentration of acids and bases through titration. It provides examples of calculating molarity from moles and volume. An experiment is described where students determine the concentration of an unknown sodium hydroxide solution by titrating it with hydrochloric acid of a known concentration.