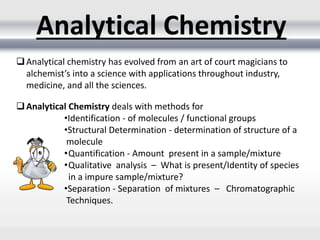
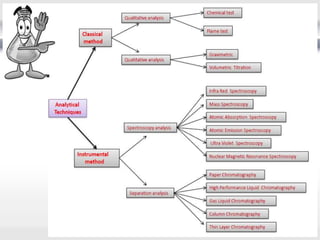
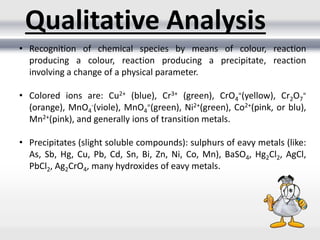
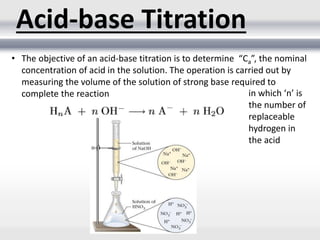
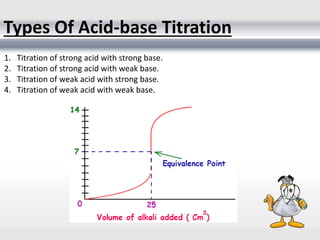
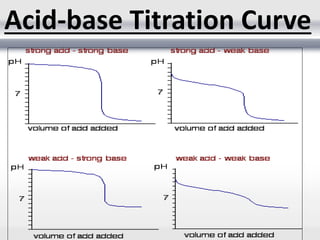
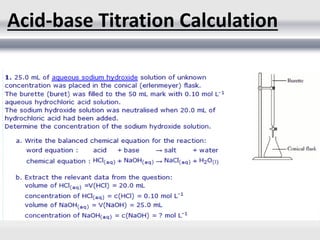
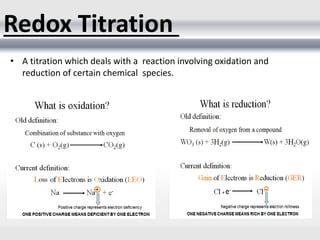
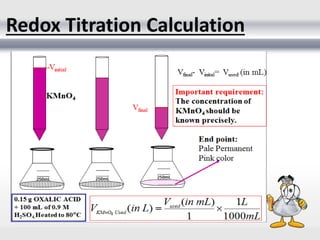
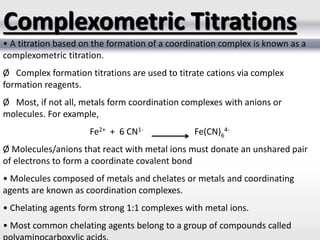
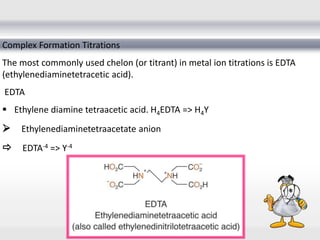
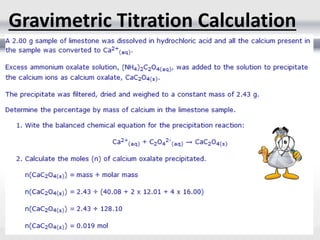
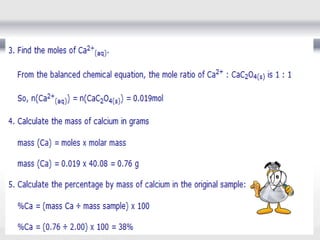
1) Analytical chemistry deals with methods for identification, structural determination, quantification, qualitative analysis, and separation of molecules and mixtures.
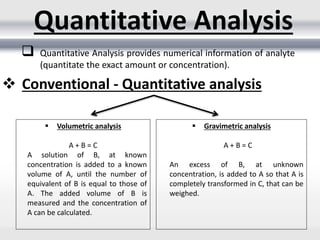
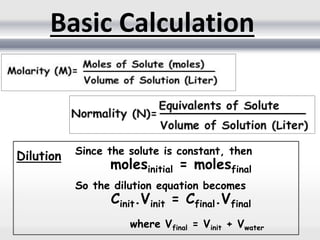
2) Quantitative analysis provides numerical information on the exact amount or concentration of an analyte and can be done through volumetric or gravimetric methods.
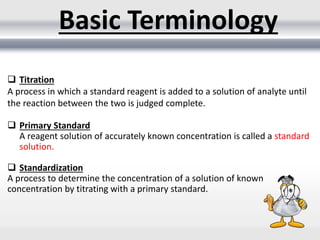
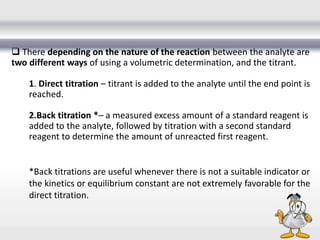
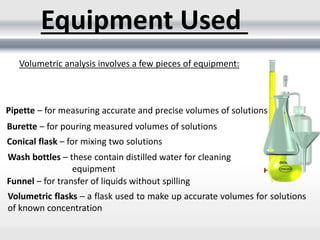
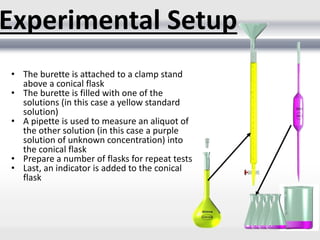
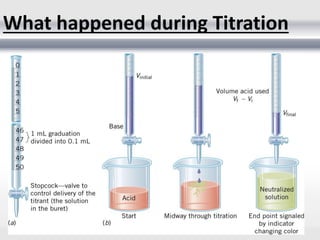
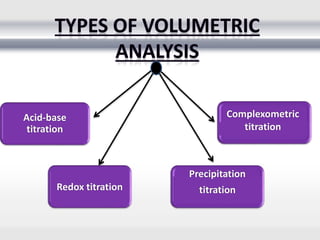
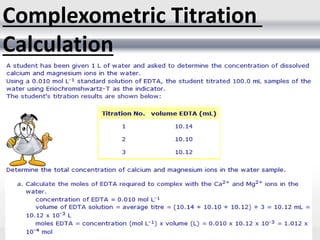
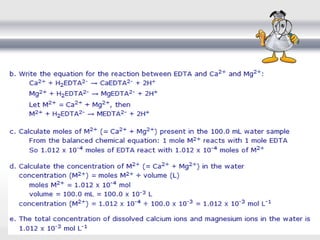
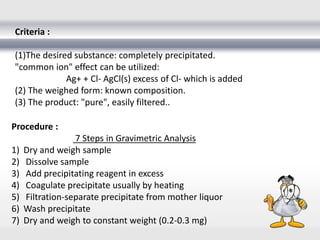
3) Volumetric analysis involves titrating a solution of known volume and concentration (titrant) with an analyte until the endpoint of the reaction is reached to determine the unknown concentration. Gravimetric analysis involves precipitating, filtering, drying, and weighing an analyte to obtain its mass concentration.