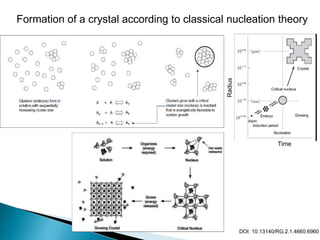
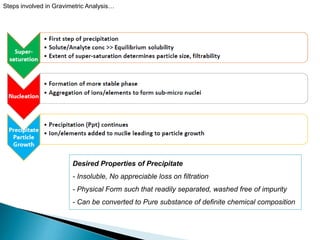
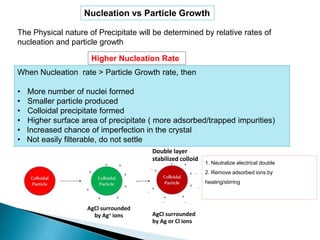
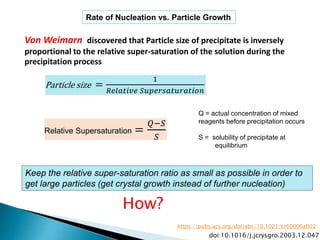
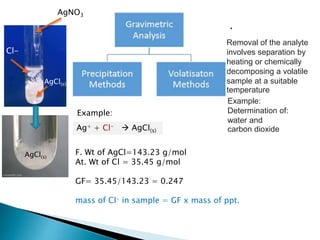
This document discusses various concepts related to gravimetric analysis methods. It covers three key points:
1) Gravimetric analysis involves selectively precipitating the analyte of interest and weighing the precipitate to determine the amount of analyte. Factors like solubility products (Ksp), common ion effects, and pH can impact precipitation.
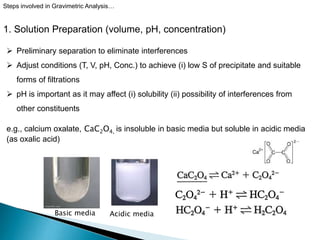
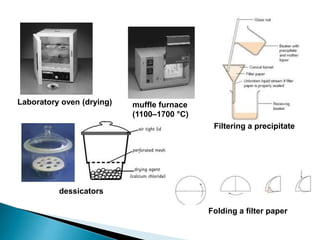
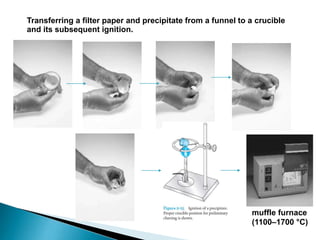
2) Key steps in gravimetric analysis are discussed, including filtering, drying, and transferring precipitates. Equipment like filters, crucibles, and drying ovens are also mentioned.
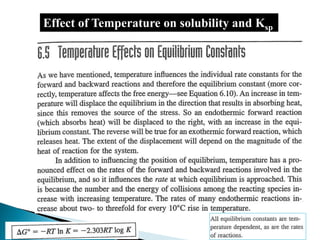
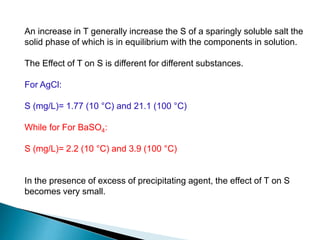
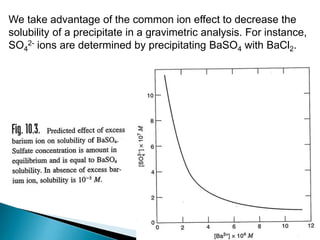
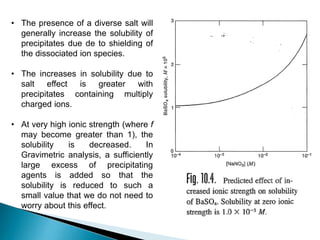
3) Solubility is impacted by various equilibrium concepts like Ksp values, common ion effects, salt effects, pH, complexation, and temperature. These concepts are illustrated through




![Solubility and Solubility Product
Solubility is the amount of solute that can be dissolved in a given
volume of solvent (g/L or mol/L) at a given temprature
Even “insoluble substances”
have slight solubility!!!
Types of electrolytes
AB type = AgCl
A2B type = Ag2CrO4
AB2 type = PBI2
It is the ionized form that determine
solubility and chemical stability
Ksp = [Ag+] [Cl-]
K = [Ag+] [Cl-] / [AgCl]
K [AgCl] = [Ag+] [Cl-]
[AgCl] const.
Ksp can be used to measure equilibrium solubility.
In calcualtions, use molL-1 for S](https://image.slidesharecdn.com/gravimetricanalysissajjadullah-190323033127/85/Gravimetric-method-of-analysis-10-320.jpg)
![Ksp = [Ag+] [Cl-]
[Ag+] = [Cl-] = S
Ksp = (S)2
S = 𝐾 𝑠𝑝
S = molar solubility
As S = 𝐾 𝑠𝑝
So S = 1x 10−10
S = 1 x 10-5 M
AB type Salt: AgCl
Example: Find S for AgCl when Ksp = 1 x 10-10
Though AgCl is a Sparingly soluble salt; it
still is a Strong electrolyte
Ksp can be used to measure equilibrium solubility.](https://image.slidesharecdn.com/gravimetricanalysissajjadullah-190323033127/85/Gravimetric-method-of-analysis-11-320.jpg)
![A2B type Salt: Ag2CrO4
Ag2CrO4 2Ag+ + CrO4
2-
S = [CrO4
2-] = 1/2 [Ag+]
Ksp = [CrO4
2-].[Ag+]2
Ksp = [CrO4
2-] . [2CrO4
2-]2
Ksp = 4. [CrO4
2-] . [CrO4
2-]2
Ksp = 4. [CrO4
2-]3
Ksp/4 = [CrO4
2-]3
[CrO4
2-] =
3 𝐾𝑠𝑝
4
𝑆 =
3 𝐾𝑠𝑝
4](https://image.slidesharecdn.com/gravimetricanalysissajjadullah-190323033127/85/Gravimetric-method-of-analysis-12-320.jpg)
![AB2 type Salt: PbI2
PbI2 Pb2+ + 2I-
S = [Pb2+] = 1
2 [I-]
Ksp =[Pb2+] . [I-]2
Ksp =1/2 [I-]3
Ksp =1/2[I-] . [I-]2
2Ksp = [I-]3
[I-] = 3
2Ksp
S =
[𝐼]
2
=
3 2𝐾𝑠𝑝
2
S =
[𝐼]
2
=
3 2𝐾𝑠𝑝
3 8
𝑆 =
[𝐼]
2
=
3 2𝐾𝑠𝑝
8
𝑎𝑠 2 =
3
8
𝑆 =
3 𝐾𝑠𝑝
4
𝑆 =
3 7.1 𝑥10^−9
4
= 1.2 x 10-3M
𝑖𝑓 𝐾𝑠𝑝 = 7.1 𝑥10−9, 𝑡ℎ𝑒 𝑆 𝑤𝑖𝑙𝑙 𝑏𝑒:](https://image.slidesharecdn.com/gravimetricanalysissajjadullah-190323033127/85/Gravimetric-method-of-analysis-13-320.jpg)
![Common Ion Effect
For the reaction
𝐴𝑔𝐶𝑙 ↔ 𝐴𝑔
+
+ 𝐶𝑙
−
The solubility of AgCl will decreases if either Cl- (or Ag+) ions
are added from some external source (say NaCl).
Ksp = 1 x 10-10
S = [Ag+] = 1 x 10-5 M
Example:
Calculate the solubility of AgCl when 2.0 mmoles (10 mL of 0.2M) of AgNO3 are
mixed with 1.0 mmole (10 mL of 0.1M) NaCl .
Solution:
mmoles of Ag+ in excess = 2-1 = 1.0 mmoles
Solubility = [Cl-]
[Ag+] = 1 mmol/20 mL = 0.05M (this concentration is very high compared to Ag
concentration, 1 x 10-5 M, in absence of a common ion. So we can neglect 1 x 10-5 M
[0.05]. [Cl-] = 1 x 10-10
[Cl-] = 1 x 10-10 / 0.05 = 2 x 10-9 M
𝐴𝑔
+
+ 𝐶𝑙
−
↔ 𝐴𝑔𝐶𝑙
Decreased S in the presence of excess Ag](https://image.slidesharecdn.com/gravimetricanalysissajjadullah-190323033127/85/Gravimetric-method-of-analysis-14-320.jpg)
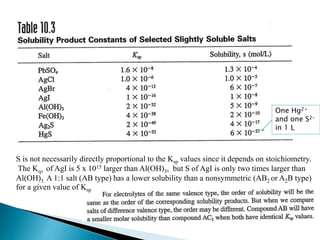
![Prediction of precipitation
For AgCl to precipitate, the product [Ag+].[Cl-] must exceed the Ksp (1 x 10-10)
value of AgCl.
Example:
What must be the concentration of added Ag+ to just start precipitation
of AgCl in a 1.0 x 10-3 M NaCl solution
[Ag+] . (1.0 x 10-3 ) = 1 x 10-10
[Ag+] = 1 x 10-7 M
[Ag+].[Cl-] = Ksp
The concentration of Ag+ must be greater than 1 x 10-7 M to start
precipitation](https://image.slidesharecdn.com/gravimetricanalysissajjadullah-190323033127/85/Gravimetric-method-of-analysis-18-320.jpg)
![Diverse Ion Effect (Salt Effect)
Since Ksp = [Ag+] [Cl-]
In the presence of an inert electrolyte (diverse salt), it is more appropriate to use
Activity (A= f.C) of ions rather concentration (C) in the equilibrium expressions
because the activity coefficient (f), and hence activity, depends on total ionic
strength.
For instance, for the case of AgCl, the K0
sp can be written as:
K0
sp = aAg+. aCl- = [Ag+]fAg+ . [Cl-] fCl-
Ksp = [Ag+] [Cl-]
Ksp =
K0
sp
fAg+ . fCl−
[Ag+] [Cl-] =
K0
sp
fAg+ . fCl−
OR
This equation shows that as activities of ions decrease (f increases), the Ksp will
increase (and so the molar solubility also increases)](https://image.slidesharecdn.com/gravimetricanalysissajjadullah-190323033127/85/Gravimetric-method-of-analysis-21-320.jpg)
![Diverse Ion Effect: Example
Calculate the Solubility of AgCl in the absence and presence of 0.1M NaNO3.
In the absence of NaNO3, (zero Ionic strength):
K0
sp = 1 x 10-10
In the presence of 0.1M NaNO3
Ksp =
K0
sp
fAg+ . fCl−
µ = ½ ∑CiZi2
µ = 0.1
fCl− = 0.76
fAg+ = 0.75
Ksp=
1x 10−10
0.75 (0.76)
= 1.8 x 10-10
New solubility= S = 𝐾 𝑠𝑝
S = 1.3 x 10-5 M (30% more than in the absence of NaNO3)
= 1.8 x 10−10
S = [Ag+] = 𝐾 𝑠𝑝 = 1 x 10-5 M](https://image.slidesharecdn.com/gravimetricanalysissajjadullah-190323033127/85/Gravimetric-method-of-analysis-22-320.jpg)

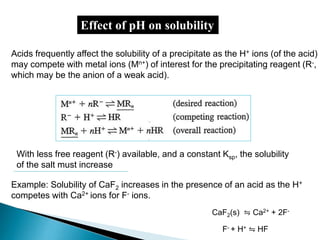
![In General, the solubility of a precipitate (MA) whose anion (A-) is derived from a weak
acid (HA) will increase in the presence of added acid (H+) because the acid will tend to
combine with the anion (A-) and thus remove the A- from solution (equilibrium shift to
RHS, leading to solubility of MA).
[A-] + [HA] = [M]
For Instance, the solubility of CaC2O4 in
the presence of acid increases as the H+
ion may take up oxalate (C2O4
2-) ions in
two steps:
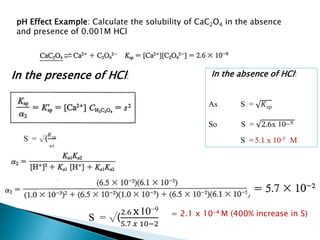
Thus we must take into account the effect of pH if we need to determine
the solubility of CaC2O4 from Ksp expression Ksp= [Ca2+].[C2O4
2-].
Solubility of CaC2O4 = Ca2+ = CH2C2O4
CH2C2O4 = [H2C2O4] + [HC2O4
-] + [C2O4
2-]
To calculate the fraction (α2) of CH2C2O4 that exist as C2O4
2-, we need
Need an expression that accounts for pH or H+ ion concentration.
(SEE NEXT SLIDE)](https://image.slidesharecdn.com/gravimetricanalysissajjadullah-190323033127/85/Gravimetric-method-of-analysis-26-320.jpg)
![Ksp= [Ca2+].[C2O4
2-].
α2=
[𝐶2
𝑂4
2
−
]
[𝐶𝐻2𝐶2𝑂4
]
=
CH2C2O4 = [H2C2O4] + [HC2O4
-] + [C2O4
2-]
Or
is called conditional solubility product and its value holds for only a specified pH](https://image.slidesharecdn.com/gravimetricanalysissajjadullah-190323033127/85/Gravimetric-method-of-analysis-27-320.jpg)
![Effect of Complexation
For example, NH3 (complexing agent) reacts with metal ion (Ag+) of the
precipitate (AgCl) to increase its solubility. For example
AgCl + 2NH3 ⇋ Ag(NH3)2
+ + Cl-
(soluble)
For a precipitate MA that dissociates to give M+ and A- and whose M+
complexes with L to form ML+, the equilibria are:
CM = [M+] + [ML+] = [A-]
Where
CM= analytical
concentration
NH3 competes with
Cl- for Ag+ ions](https://image.slidesharecdn.com/gravimetricanalysissajjadullah-190323033127/85/Gravimetric-method-of-analysis-29-320.jpg)
![Ksp= [Ag+] [Br-] = 4 x 10-13
Consider the effect of NH3 on solubility of AgBr.
S =[Br-] = CAg
CAg= [Ag+] + [Ag(NH3)+] +[Ag(NH3)2
+]
We can substitute CAg.β0 for [Ag+]
in the Ksp relation:
β0 is the fraction of
silver species that
exists as Ag+
β0 = [Ag+]
CAg
s2= ks 𝑃
β0
s= ks 𝑃
β0
OR](https://image.slidesharecdn.com/gravimetricanalysissajjadullah-190323033127/85/Gravimetric-method-of-analysis-30-320.jpg)
![Example: Calculate the molar solubility of AgBr in the
absence and presence of 0.1M NH3 solution
In the Absence of NH3 In the Presence of NH3
S = 𝐾 𝑠𝑝
So, S = 4x 10−13
S = 6.3 x 10-7 M
Ksp= [Ag+] [Br-] = 4 x 10-13
β0 for 0.1M NH3= 4 X 10-6
s= ks 𝑃
β0
s= 4 𝑥10
−
13
4 x 10−6
s= 3.2 x 10-4 M
(530 times more soluble in
the presence of NH3)](https://image.slidesharecdn.com/gravimetricanalysissajjadullah-190323033127/85/Gravimetric-method-of-analysis-31-320.jpg)
![Other examples
AgNO3 (aq) + KCN (aq ⇋ AgCN(s) + KNO3 (aq
However, if excess of KCN is added, the AgCN precipitate dissolves due to the
formation of soluble [Ag(CN)2]- complex ion.
AgCN precipitates as the Ksp value of AgCN is exceeded by addition of KCN to
AgNO3 solution:
AgCN(s) + KCN (aq ⇋ [Ag(CN)2]-
(aq) + KNO3 (aq
(soluble)
Al3+
(aq) + OH-
(aq ⇋ Al(OH)3(s)
In the presence of F- ions (that compete with OH- ion), the solubility of Al(OH)3
increases due to the formation of [AlF6]3- ions.
Al(OH)3(s) + 6F-
(aq ⇋ Al(F)6 ]3-
(aq)](https://image.slidesharecdn.com/gravimetricanalysissajjadullah-190323033127/85/Gravimetric-method-of-analysis-32-320.jpg)