This document discusses various types of redox titrations and indicators used. It describes the preparation and standardization of common redox titrants like potassium manganate(VII), iodine, potassium dichromate, potassium bromate and ceric ammonium sulfate. Examples of titrations included are standardization of KMnO4 with sodium oxalate or sodium thiosulfate, iodine with sodium thiosulfate or arsenic trioxide, and sodium thiosulfate with potassium iodate. The document also covers redox indicators and conditions for iodometric titrations.




![Standardisation of KMnO4 With sodium oxalate
• Method : Dissolve accurately weighed 6.7 g of sodium oxalate previously dried at
110oC in water and then make up volume to 1 litre. Pipette out 20 ml of this
solution in conical flask, add 5 ml of concentration H2SO4 and then warm upto
70C. Titrate against KMnO4 until pink colour persist for 30 s.
PRECAUTIONS DURING STANDARDISATION OF KMNO4:
1. Clean the flask with concern H2SO4 or hydrogen peroxide after each titration.
2. To high temperature leads to formation of brown color solution due
decomposition of KMnO4 into MnO2.
3. Insufficient acid leads to formation of MnO2 which again gives brown color to
solution.
REACTION FOR SODIUM OXALATE :
• Oxidising agent : 2[MnO + 8H+ + 5e– –– Mn+2]
• Reducing agent : 5[(COONa)2 (COOH)2 –– 2CO2 + 2H+ + 2e–]
Overall redox balance reaction is :
2KMnO4 + 5Na2C2O4 + 5H2SO4 –– K2SO4 + 2MnSO4 + 10 CO2 + 5Na2SO4 + 8H2O.
2KMnO4 = 5Na2C2O4= 10e–](https://image.slidesharecdn.com/oxidation-reductiontitration-181026044732/85/Oxidation-reduction-titration-5-320.jpg)
![• Standardisation of KMnO4 with arsenic trioxide :
• A sample of pure arsenic trioxide is titrated in
acidic medium with KMnO4solution. KMnO4 act as self
indicator. Arsenic trioxide is dissolved in water to give
arsenious acid which is oxidised by KMnO4 solution.
The oxidation of arsenious oxide by KMnO4 does not
proceed rapidly at room temperature so catalyst like
iodide, iodate or iodine is used so reaction occur
quickly and titration is possible at room temperature.
• Reaction : As2O3 + 3H2O –– 2H3AsO3
• Oxidising agent : 2[MnO4 + 8H+ + 5e––– Mn+2]
• Reducing agent : 5 [H3AsO3 + H2O –– H3AsO4 + 2H+
+ 2e]
2KMnO4=5H3AsO3= 10 e–](https://image.slidesharecdn.com/oxidation-reductiontitration-181026044732/85/Oxidation-reduction-titration-7-320.jpg)
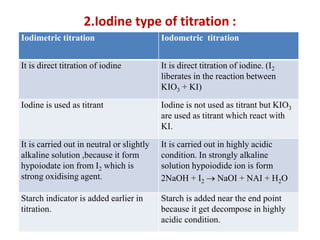
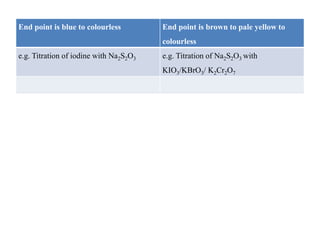
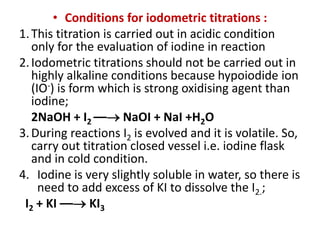


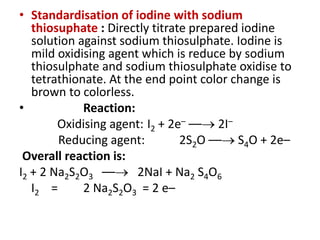
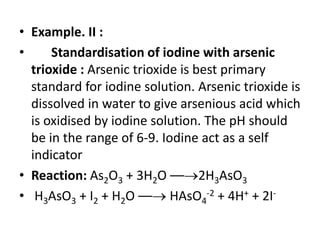
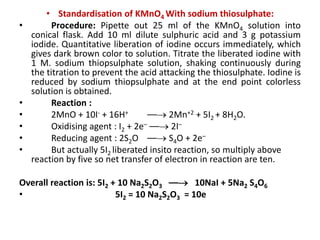
![• Example. I : Iodometry
Standardisation of sodium thiosuphate with potassium iodate :
Dissolve accurately weighed 1.3 g of pure and dry potassium iodate
into 250 ml distilled water. Pipette out 25 ml of solution into conical
flask, add 2 g potassium iodide and dilute sulphuric acid 4 ml iodine
liberate in reaction, titrate the liberated iodine with 0.1 M sodium
thiosuphate solution. When the colour of the solution becomes
pale yellow (near to end point) add starch paste as an indicator.
Continue titration until the solution become colourless.
• Reaction :
• IO + 5I– + 6H+ –– 3I2 + 3H2O
• Oxidising agent: 3[I2 + 2e– –– 2I–]
• Reducing agent: 3 [2S2O –– 2e– + S4O]
• Overall reaction is: 3[I2 + 2 Na2S2O3 –– 2 NaI + Na2 S4O6]
• 3I2 = 6 Na2S2O3 = 6e–](https://image.slidesharecdn.com/oxidation-reductiontitration-181026044732/85/Oxidation-reduction-titration-15-320.jpg)
![Potassium Dichromate Titration :
(a) Preparation of 1N K2Cr2O7 : Dissolve 49.036 g equivalent K2Cr2O7 of in 1000
ml of water to prepare 1 N solution of K2Cr2O7.
(b) Method: Accurately weighted 0.21 g potassium dichromate (K2Cr2O7) is
dissolved in 100 ml water, add 3 g Potassium iodide and acidify the solution
with 5 ml sulphuric acid. The reaction between iodide and potassium
dichromate in acidic condition liberate iodine, which is then titrated with
sodium thiosulphate using starch paste as an indicator. End point is Blue to
colourless.
Reaction :
• K2Cr2O7 + 6KI + 7H2SO4 –– Cr2 (SO4)3 + 3I2 + 4K2SO4 + 7H2O
• Oxidising agent: 3[I2 + 2e– –– 2I–]
• Reducing agent: 3 [2S2O3 2e– + S4O6]
Overall reaction is: 3[I2 + 2 Na2S2O3 –– 2 NaI + Na2 S4O6]
3I2 = 6 Na2S2O3 = 6e–](https://image.slidesharecdn.com/oxidation-reductiontitration-181026044732/85/Oxidation-reduction-titration-16-320.jpg)
![• Potassium Bromate Titration :
Sodium thiosuphate is standardised by using potassium bromate as
primary standard. It is a type of iodometric titration.
• Method:
Accurately weighted 1.3 g of KBrO3 is dissolved in water, add
hydroiodic acid in solution it react with KBrO3 and form IO3
-. Add in
above solution 2 g of Potassium iodide and acidify the solution with
4 ml dil. HCl and titrate with Na2S2O3 using starch paste as an
indicator.
Reaction :
KBrO3 + HI –– HIO3 + KBr
6H+ + IO3
- + 5I– –– 3I2 + 3H2O
Oxidising agent: 3[I2 + 2e– –– 2I–]
Reducing agent: 3 [2S2O –– 2e– + S4O]
Overall reaction is: 3[I2 + 2 Na2S2O3 –– 2 NaI + Na2 S4O6]
3I2 = 6 Na2S2O3 = 6e–](https://image.slidesharecdn.com/oxidation-reductiontitration-181026044732/85/Oxidation-reduction-titration-17-320.jpg)







