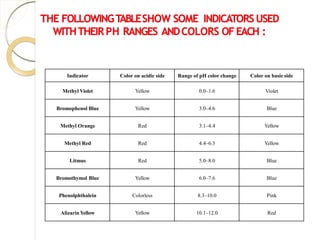
The document outlines techniques in volumetric analysis, focusing on methods such as titration for determining the concentration of unknown solutions. It discusses key concepts like standard and unknown solutions, the titration process, types of titrations, and various methods for detecting endpoints. Additionally, it highlights the importance of accuracy, precision, and reliability in pharmaceutical analysis.









![Limitations
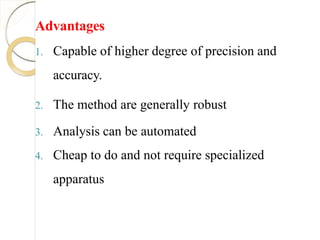
1. Non selective
2. Time consuming if not automated and
require greater level of operator skill
3. Require large amount of sample
4. Reaction of standard solution should be rapid
and complete 1]](https://image.slidesharecdn.com/titrimetricanalysis-190916112107/85/Titrimetric-analysis-16-320.jpg)