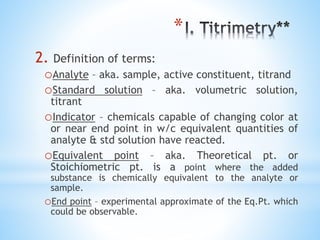
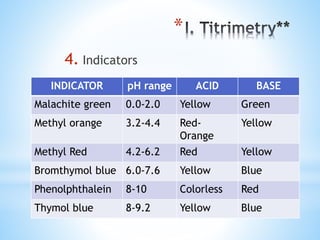
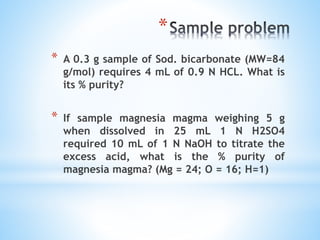
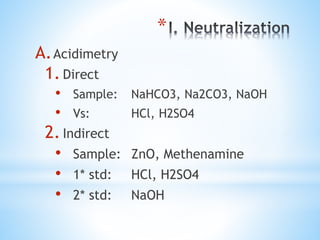
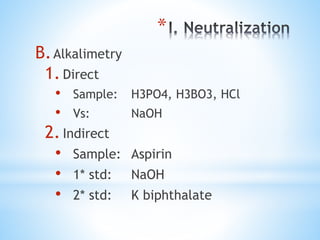
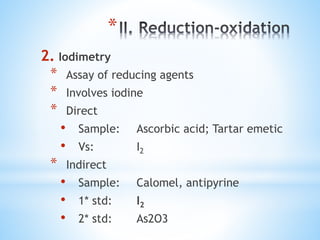
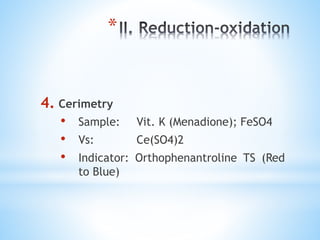
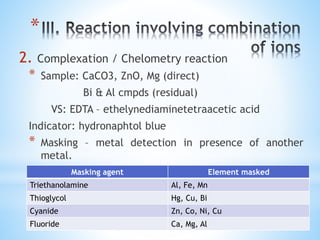
This document discusses various topics in pharmaceutical analysis including qualitative and quantitative analysis, classification of analytical methods based on sample size and nature of method, pH and buffer capacity calculations, and different titration methods such as acidimetry, alkalimetry, permanganometry, and complexometric titrations. Key analytical techniques discussed include titrimetry, gravimetry, spectrometry, electrochemistry, and chromatography.








![*
1.Negative logarithm of molar
concentration of Hydrogen ion;.
2.pH = -log[H+] or pH = log
1
[𝐻]
3.Henderson-Hasselbalch equation
opH = pka + log
[𝑠𝑎𝑙𝑡]
[𝑤𝑒𝑎𝑘 𝑎𝑐𝑖𝑑]
or
opH = pka + log
[𝑐𝑜𝑛𝑗𝑢𝑔𝑎𝑡𝑒 𝑏𝑎𝑠𝑒]
[𝑤𝑒𝑎𝑘 𝑎𝑐𝑖𝑑]
•pKa – acid dissociation constant](https://image.slidesharecdn.com/qc-1review-160923234729/85/Qc-1-review-10-320.jpg)


![*
1. Ability of buffer solution to resist
change in pH upon addition to
acid/alkali.
2. BC equation =
[𝑠𝑡𝑟𝑜𝑛𝑔 𝑎𝑐𝑖𝑑 𝑜𝑟 𝑏𝑎𝑠𝑒]
Δ 𝑝𝐻
3. Van slyke:
Amount in g/L of strong acid or a strong
base required to be added to a solution to
change it’s pH by 1 unit.](https://image.slidesharecdn.com/qc-1review-160923234729/85/Qc-1-review-13-320.jpg)