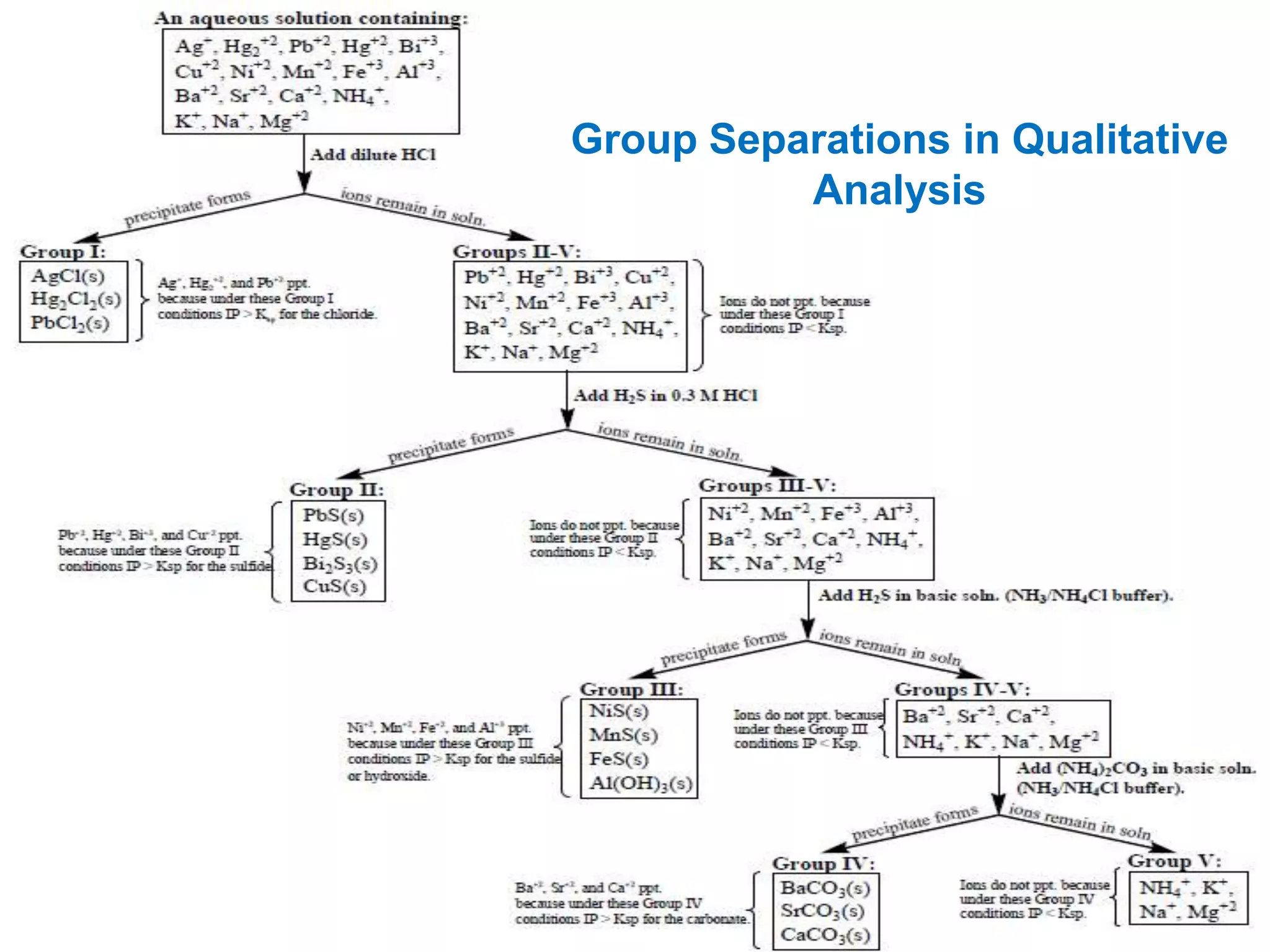
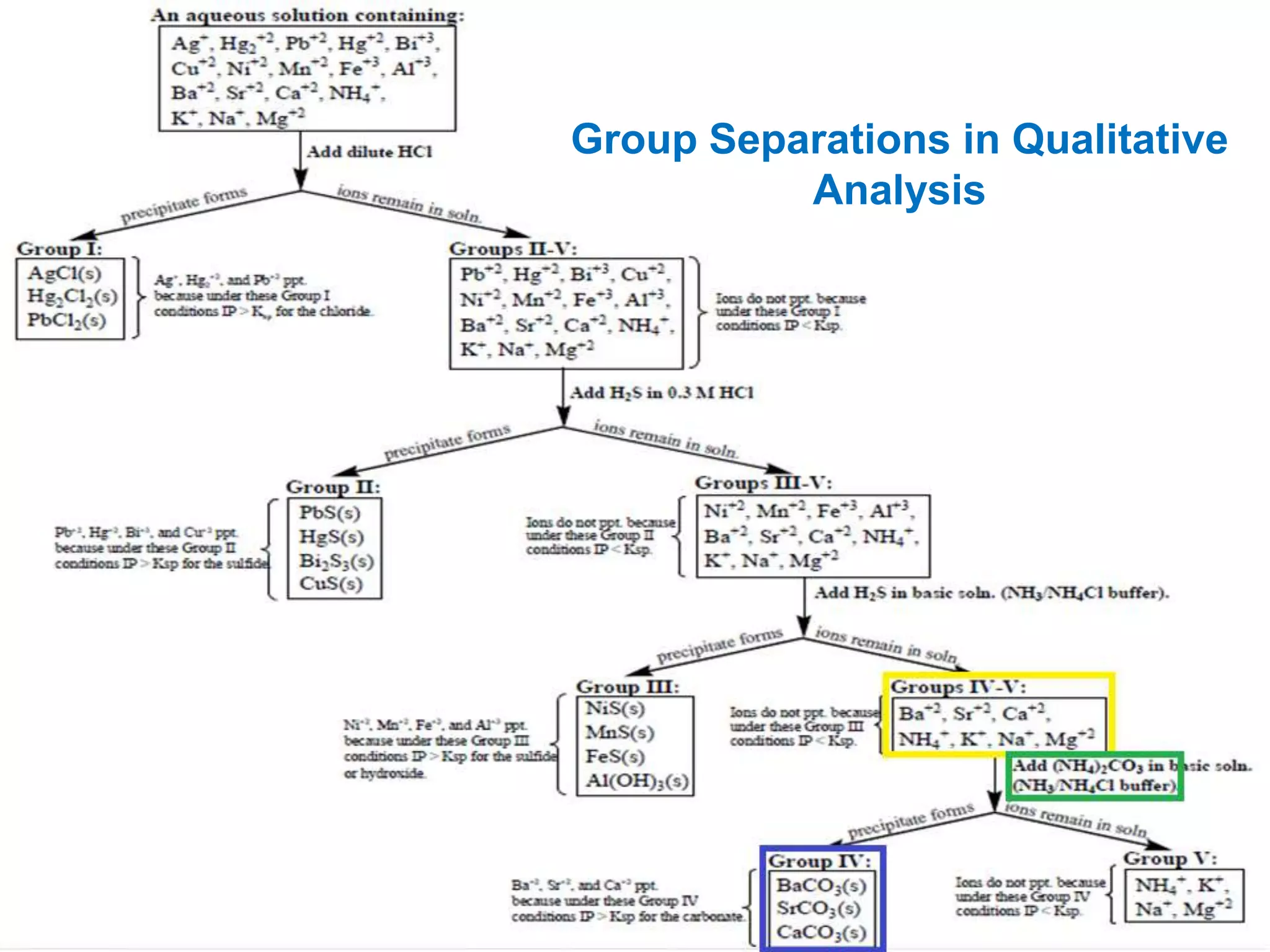
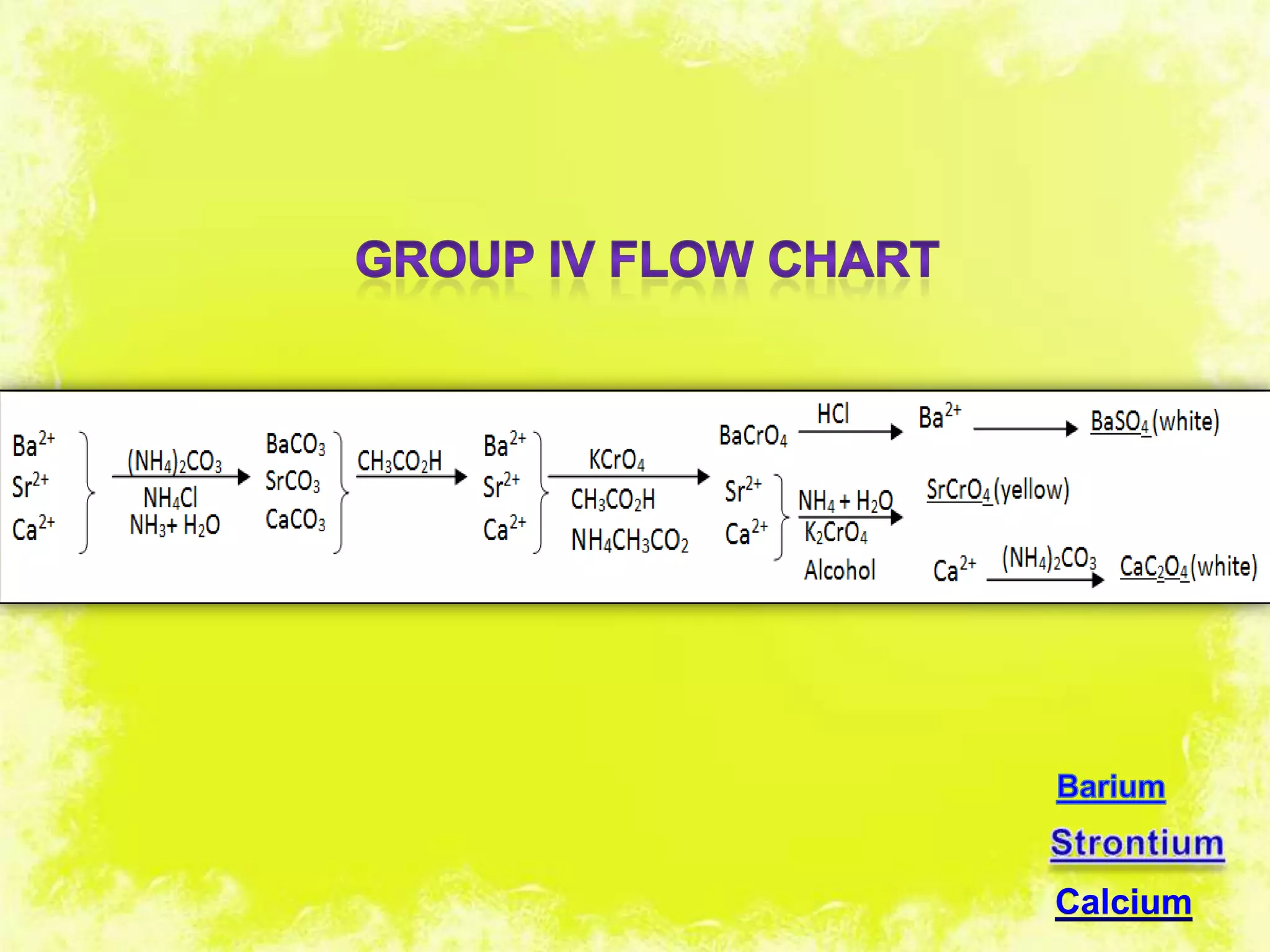
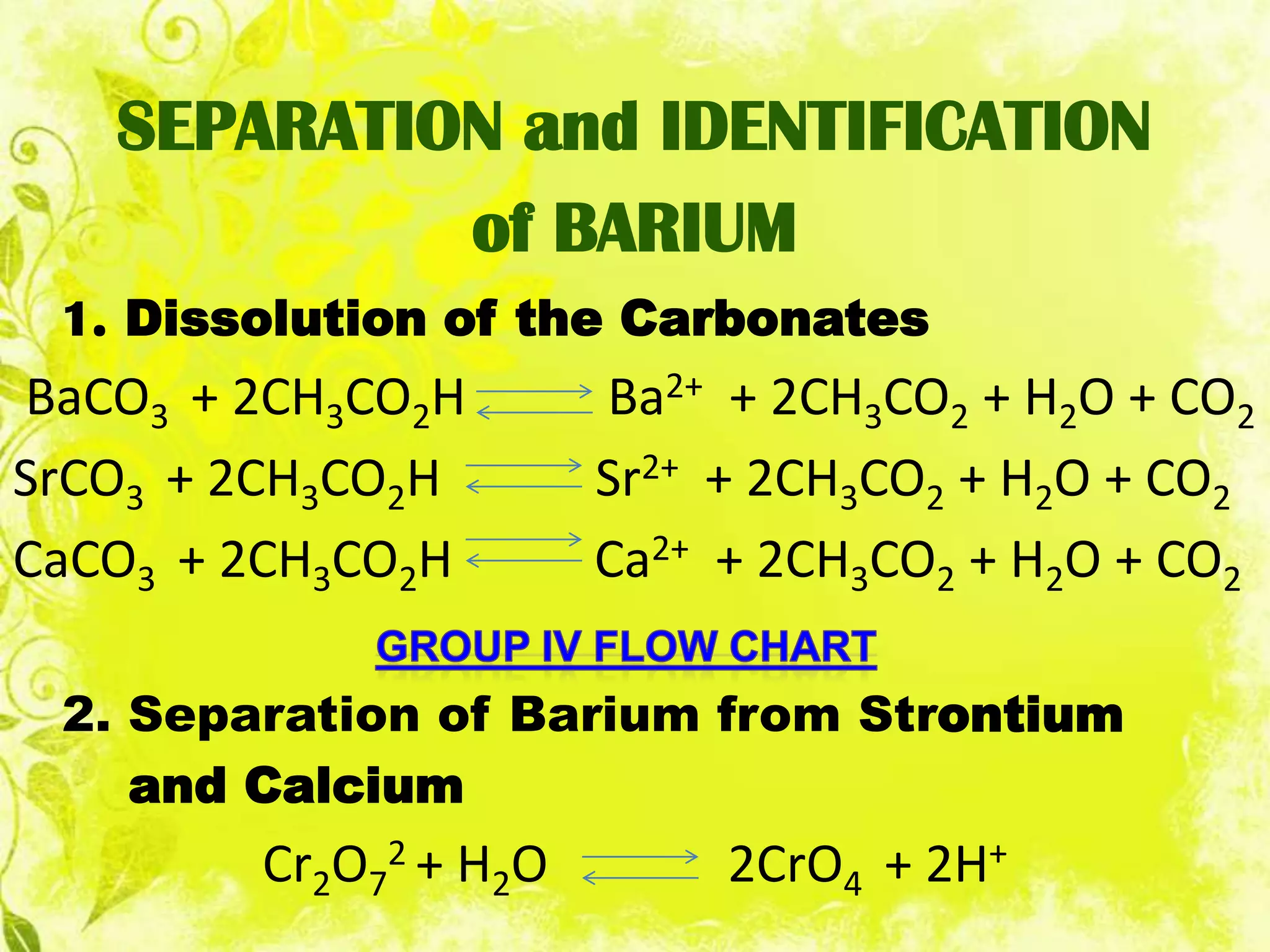
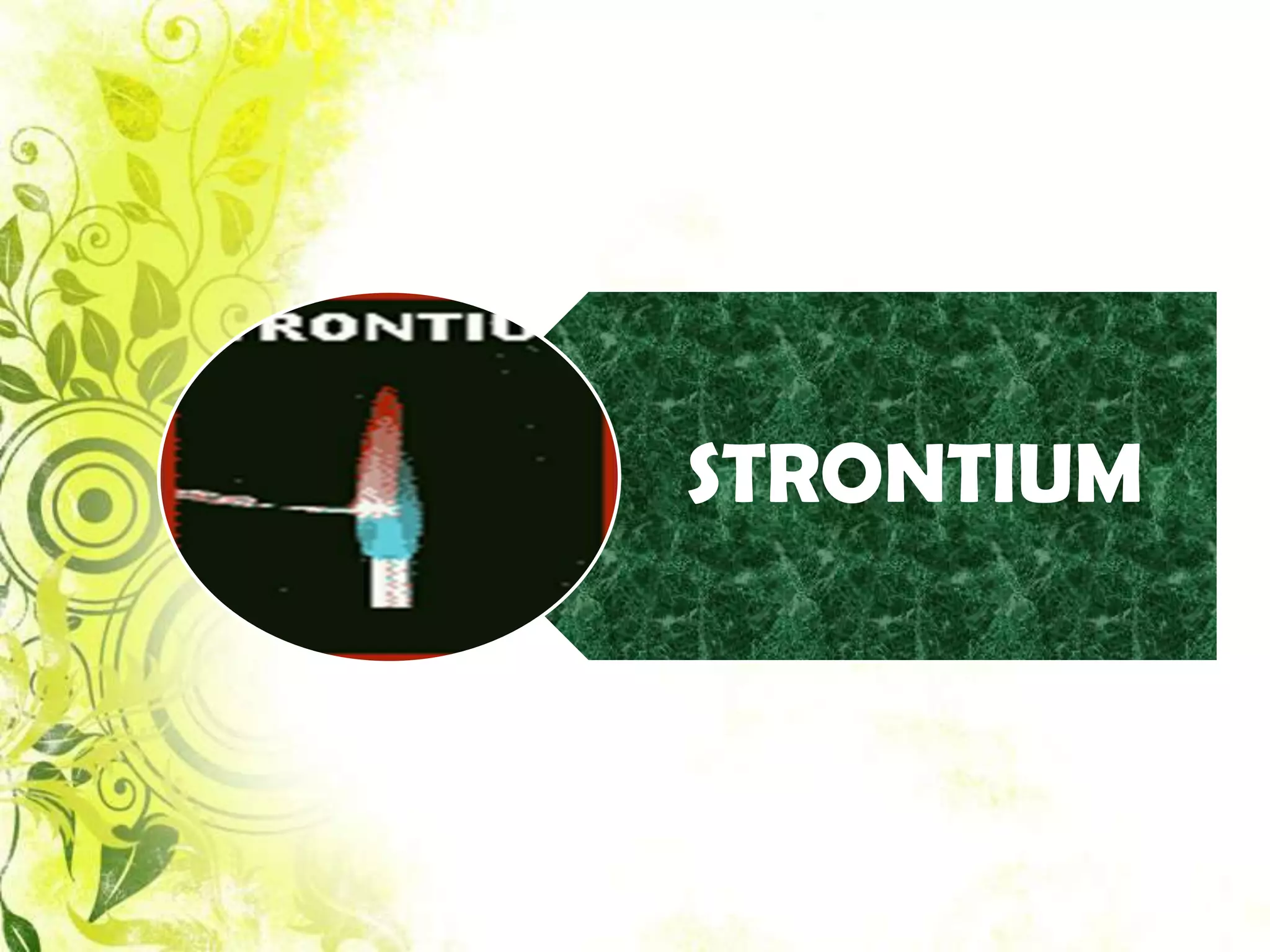
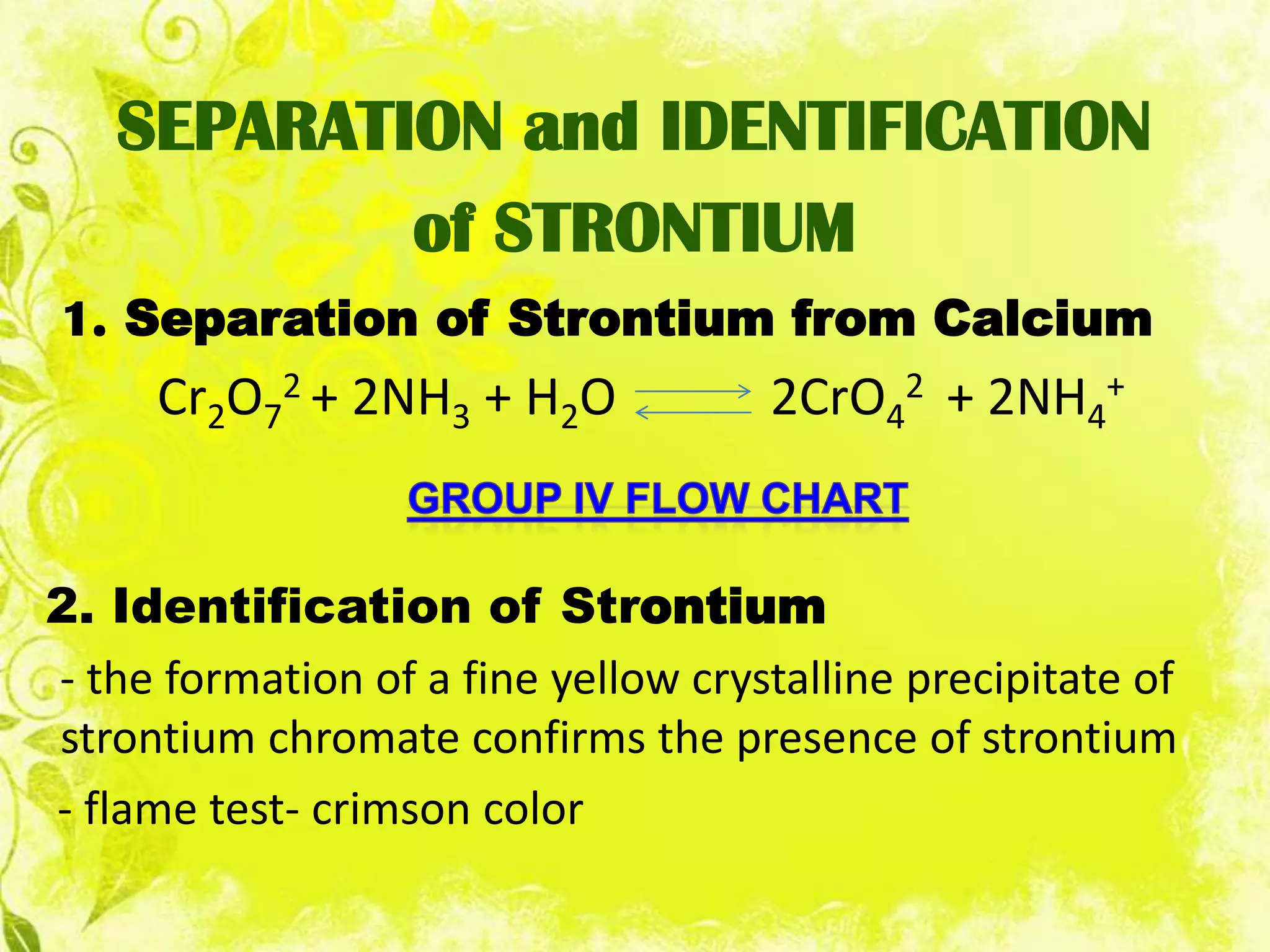
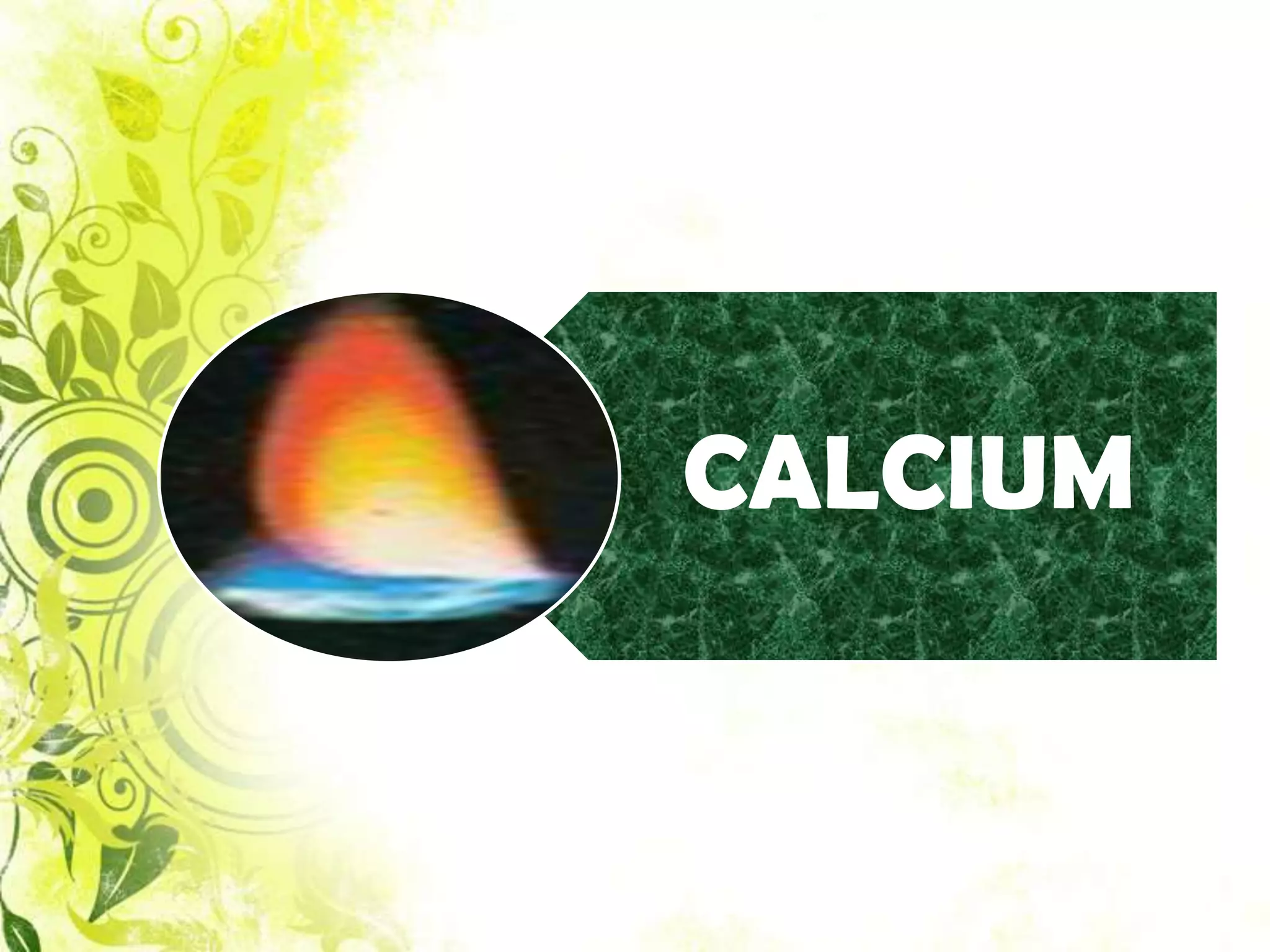
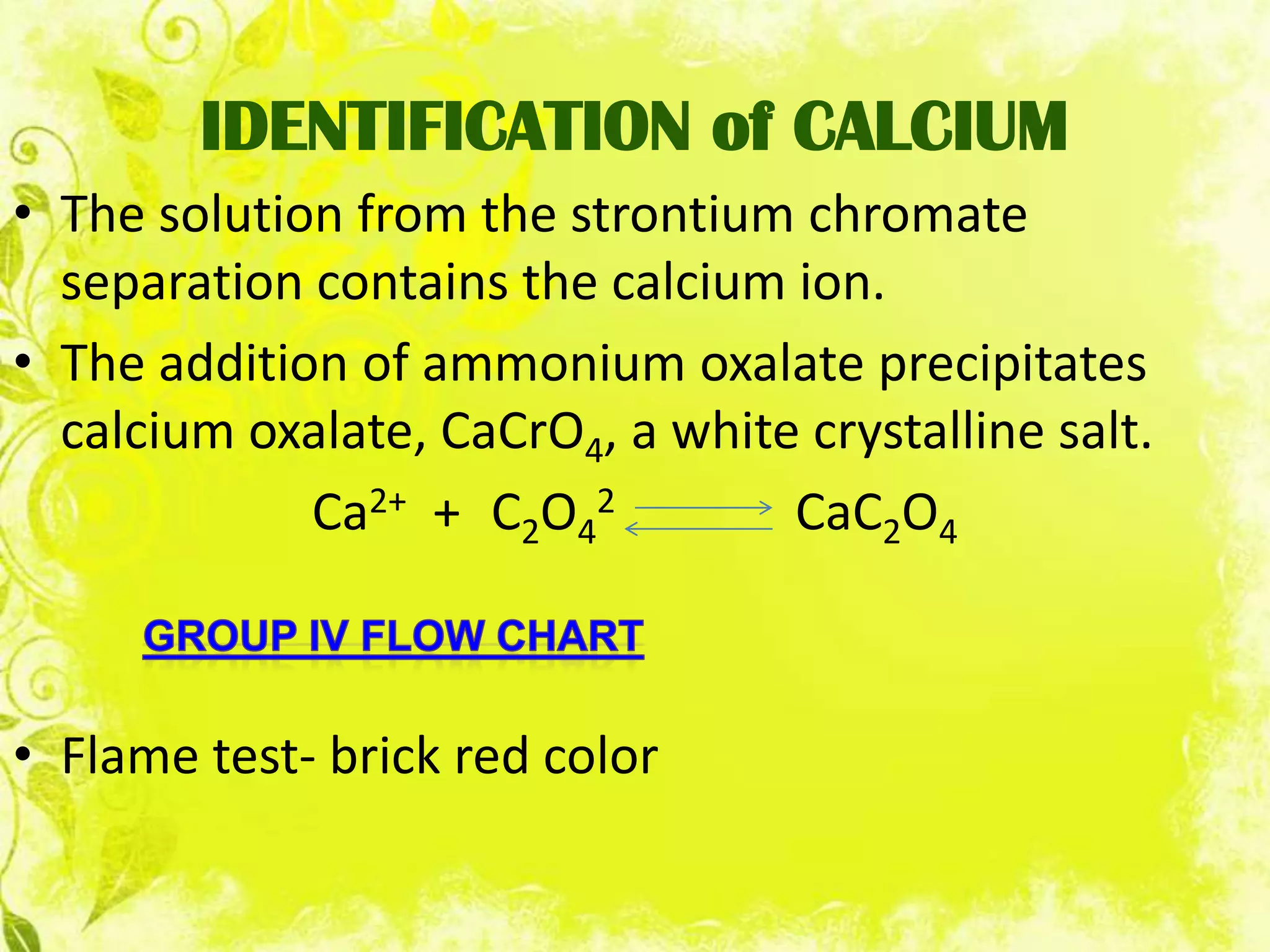
This document analyzes group IV cations including barium, strontium, and calcium. It discusses how they form precipitates with carbonates in ammonium solutions and outlines their separation and identification processes. Barium, strontium, and calcium carbonates precipitate out of solution and can then be dissolved with acetic acid. Barium is identified through precipitation of barium chromate and barium sulfate. Strontium is identified by precipitation of strontium chromate which is yellow. Calcium is identified by precipitation of calcium oxalate which is white.