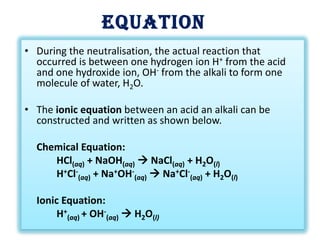
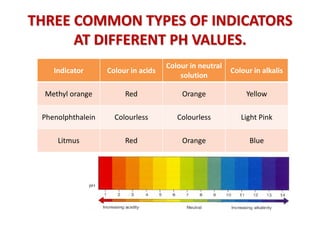
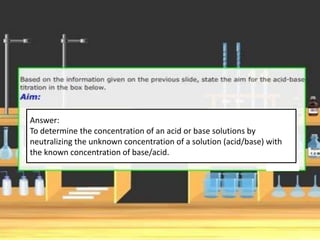
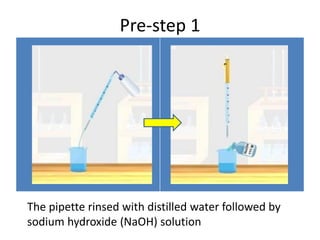
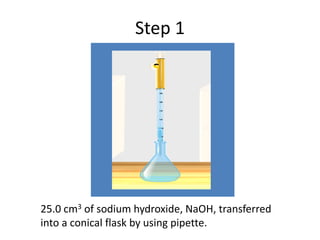
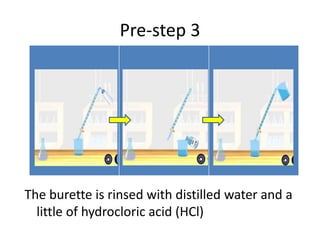
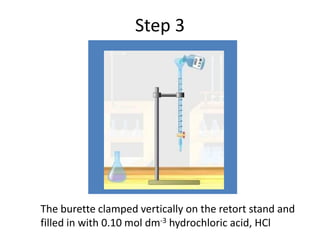
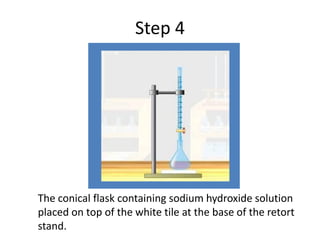
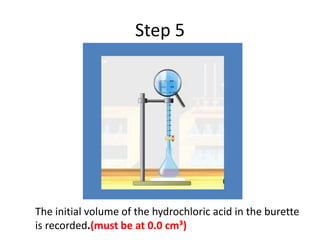
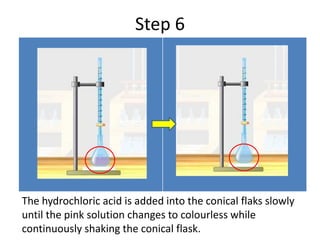
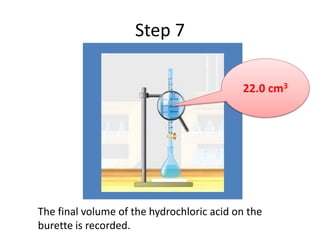
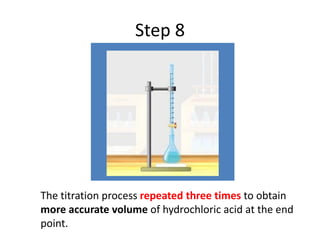
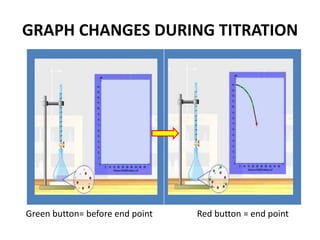
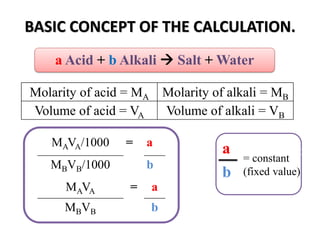
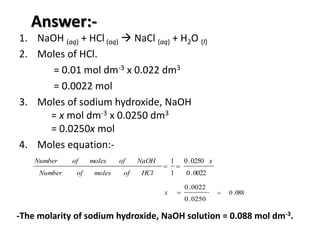
Acid-base titration is a technique used to determine the concentration of an acid or base by neutralizing a solution of unknown concentration with a solution of known concentration. The reaction is monitored using an indicator that changes color at the endpoint of the titration. The document outlines the basic procedure which involves transferring a sample of the solution of unknown concentration into a flask, adding an indicator, and then titrating with the solution of known concentration until the endpoint is reached as indicated by the color change of the indicator. The volume added is used to calculate the concentration of the original unknown solution.