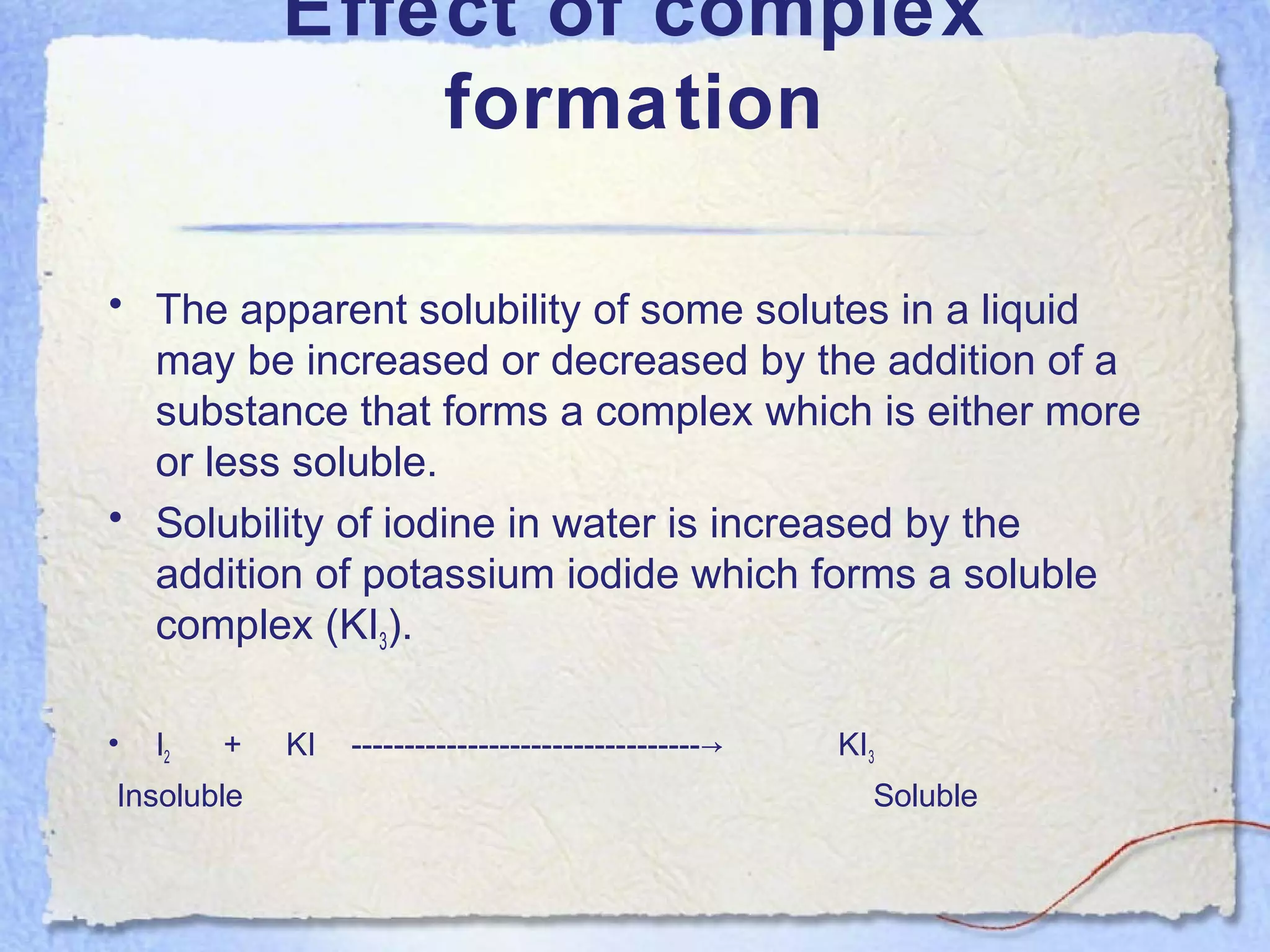
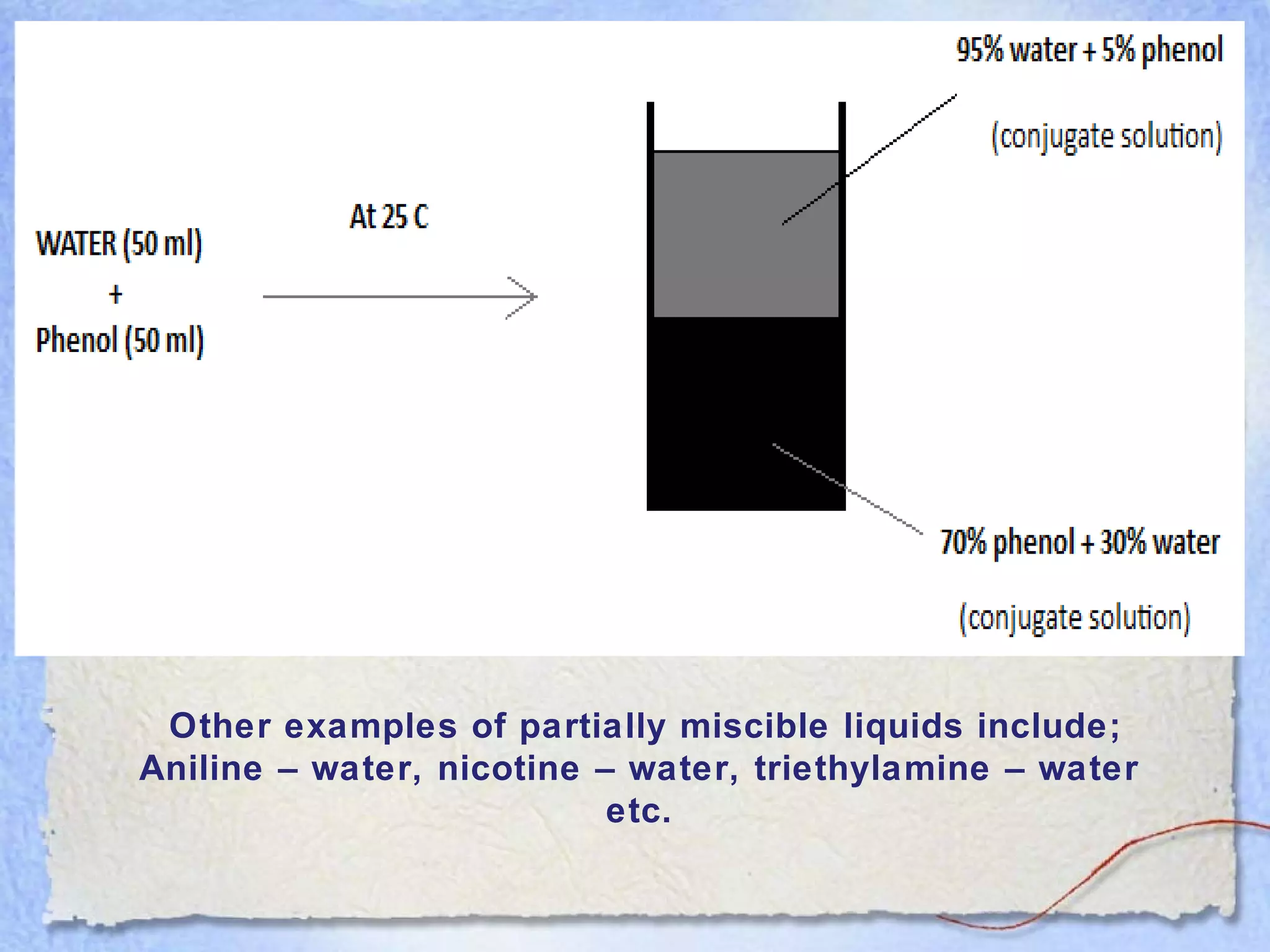
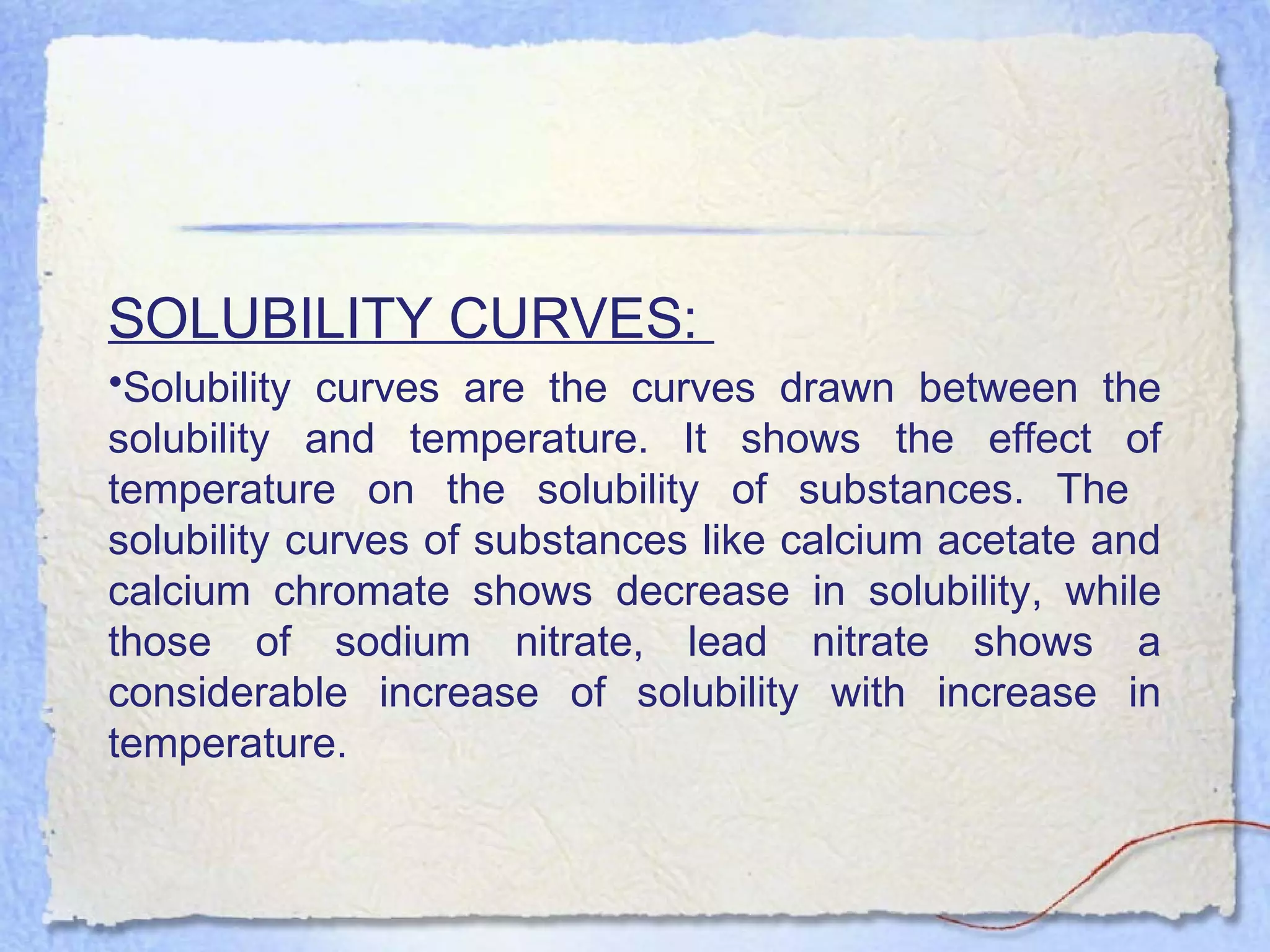
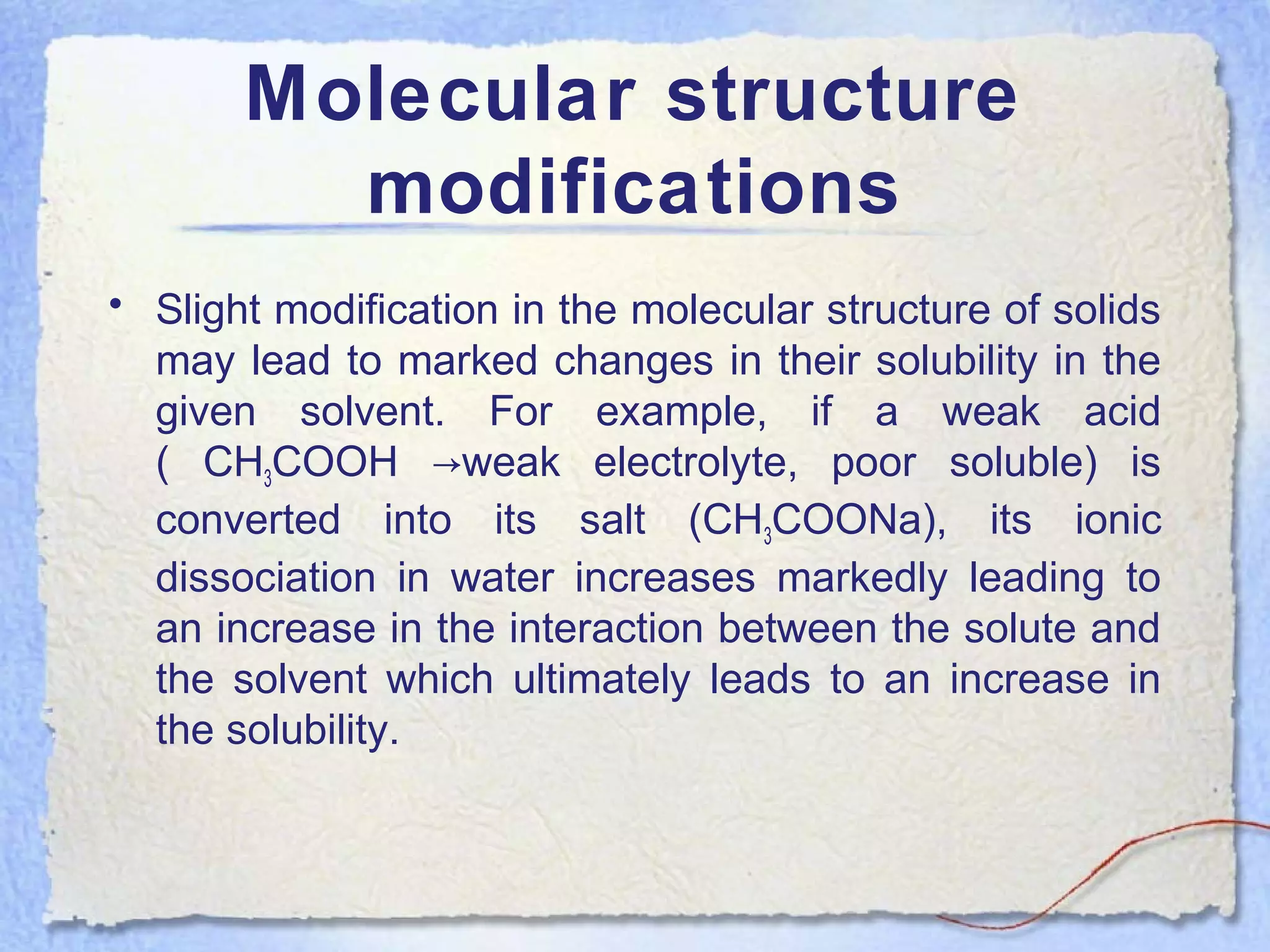
Solubility is the property of a substance to dissolve in a solvent to form a homogeneous solution. The solubility of a substance depends on factors like temperature, pressure, pH, particle size, and molecular structure. Temperature generally has a direct relationship with solubility - as temperature increases, solubility increases for most substances but decreases for those that dissolve with heat evolution. Adding electrolytes can decrease the solubility of non-electrolytes through the common ion or salting out effect. Surfactants and complex formation can increase solubility. Gas solubility increases with pressure according to Henry's law.










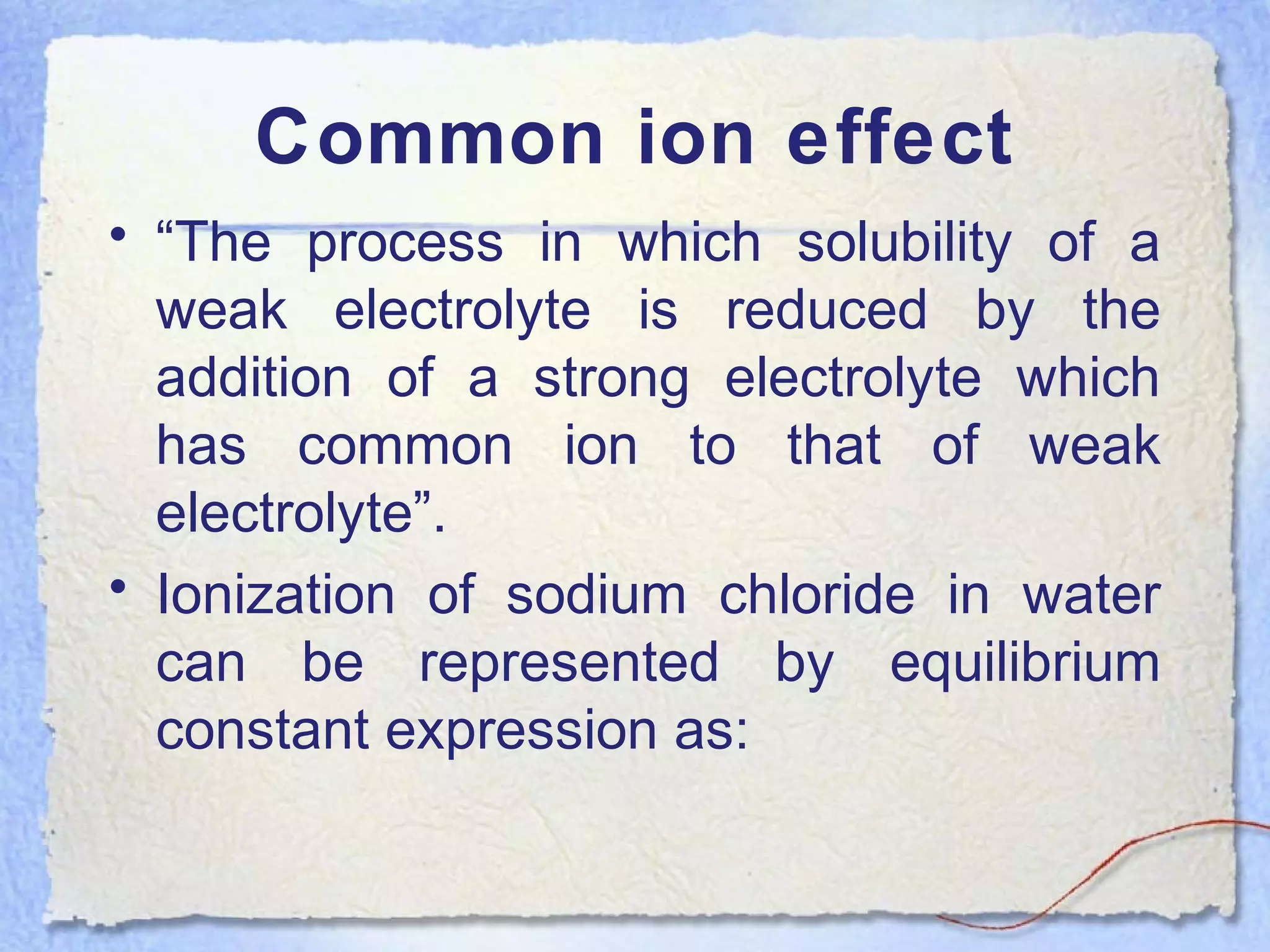
![NaCl (SOLID) Na+↔ (aq ) + Cl -
(aq)
Kc = [Na+] [Cl-
] / [NaCl]
HCl ionizes in water as:
HCl H+↔ (aq) + Cl-
(aq)
•On passing HCl gas through aqueous solution of
NaCl , concentration of Cl-
ions is increased,
therefore some of the NaCl is precipitated out to
maintain the constant value of the equilibrium
constant. This is called as common ion effect
which reduces solubility.](https://image.slidesharecdn.com/solubility-160329123327/75/Solubility-16-2048.jpg)