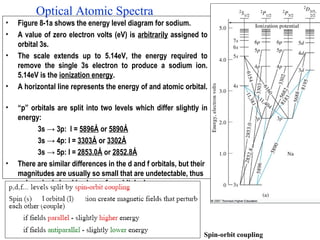
Spectrophotometry and colorimetry are concerned with measuring light absorption and transmission in solutions. Spectrophotometry covers the ultraviolet, visible, and infrared regions of the electromagnetic spectrum, while colorimetry focuses on the visible region. Beer's Law states that the absorbance of light is directly proportional to the concentration of an absorbing substance in solution. Absorbance follows the Beer-Lambert Law, which relates the intensity of transmitted light to properties of the absorbing medium. Atomic absorption spectrometry and atomic emission spectrometry are analytical techniques that use either absorption or emission spectroscopy to measure the concentration of elements in solutions.