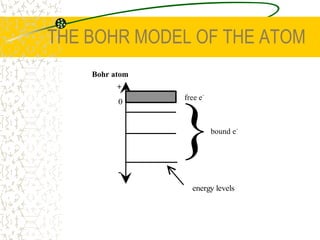
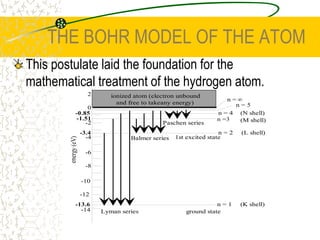
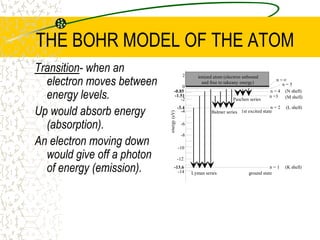
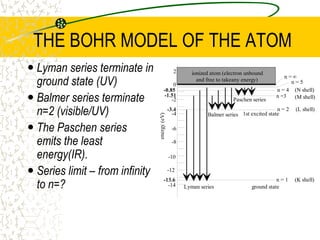
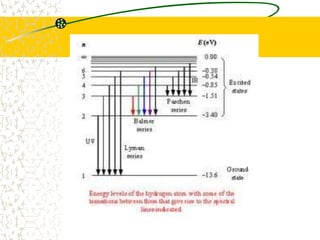
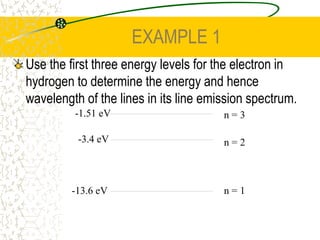
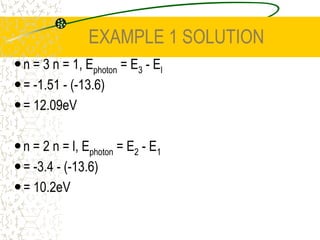
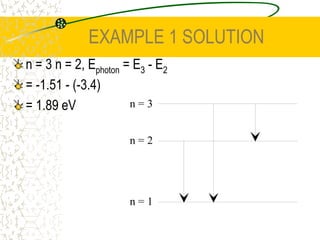
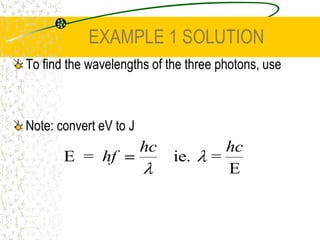
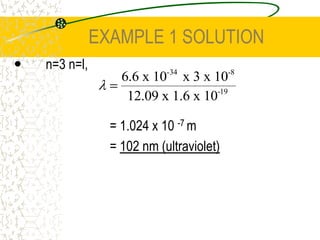
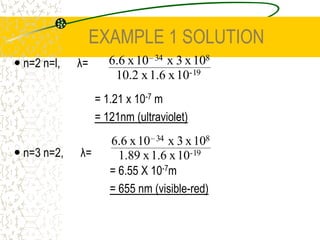
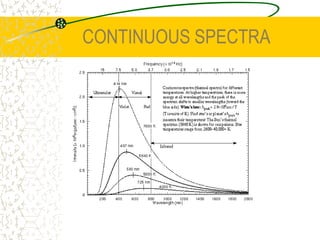
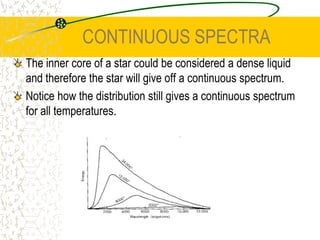
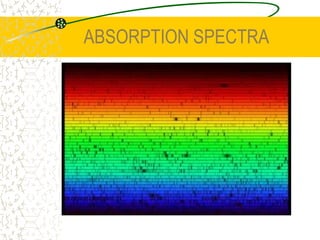
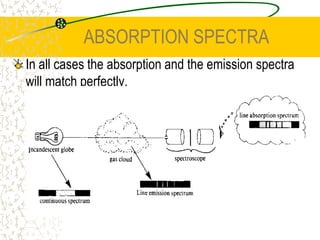
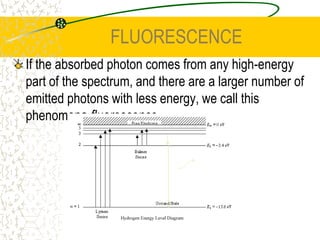
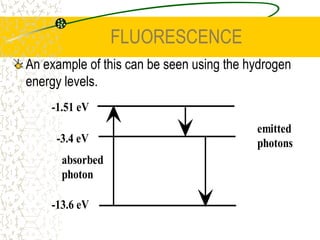
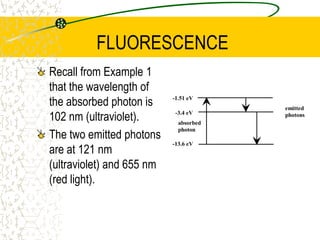
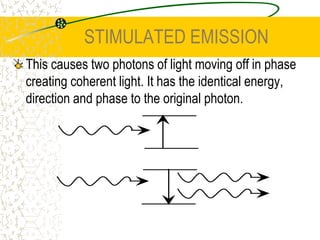
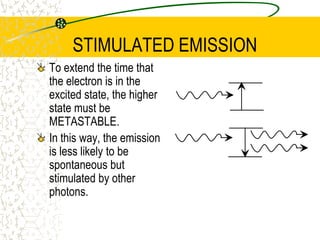
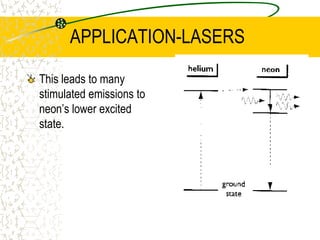
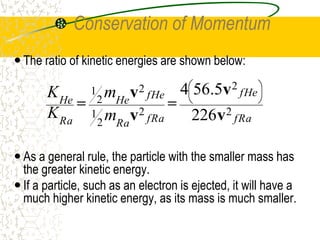
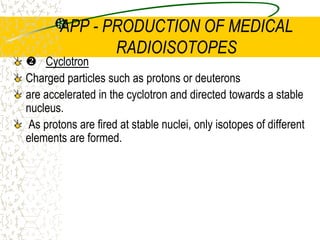
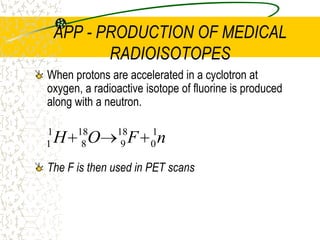
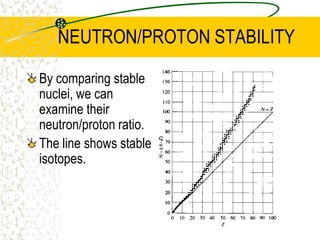
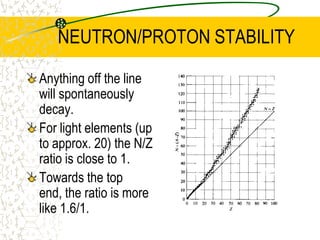
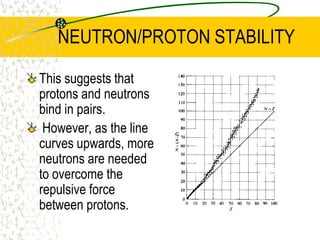
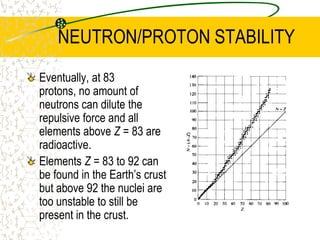
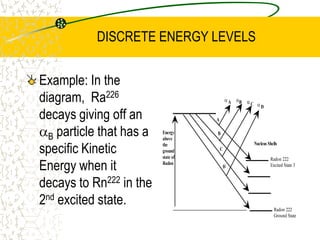
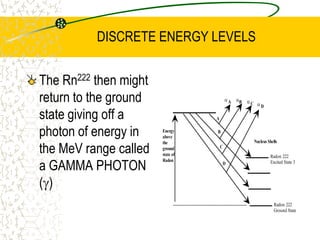
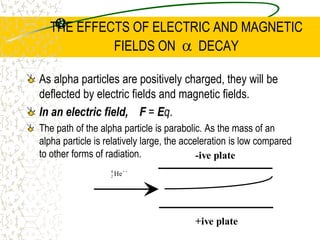
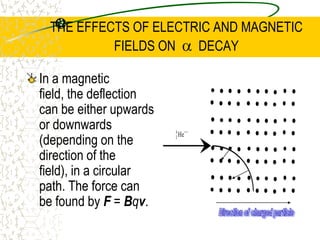
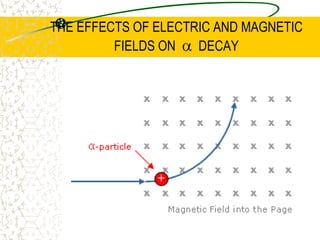
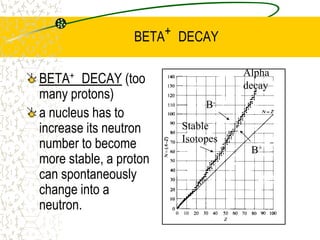
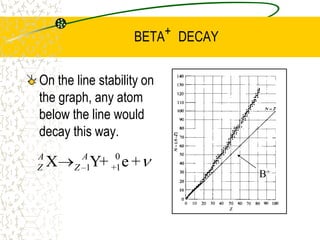
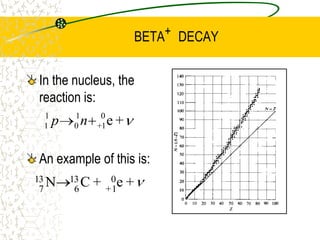
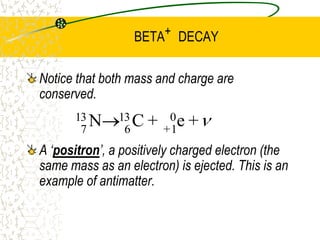
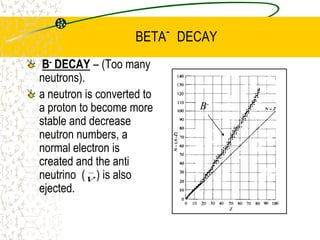
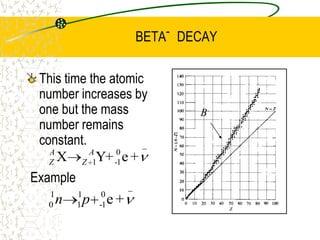
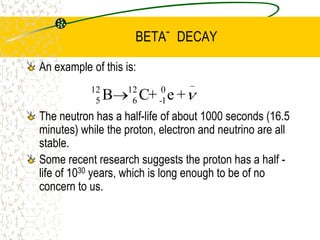
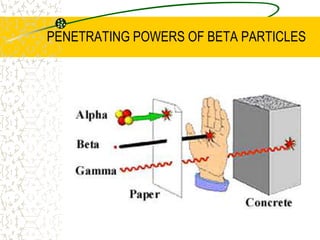
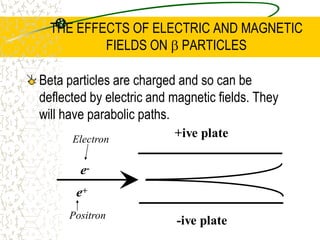
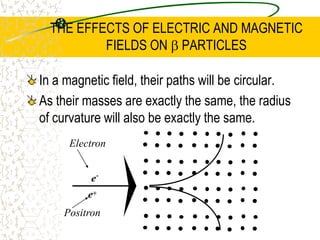
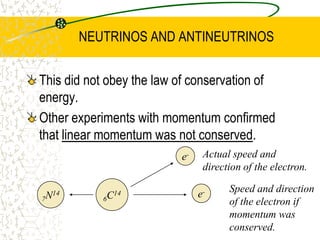
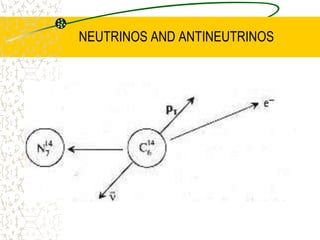
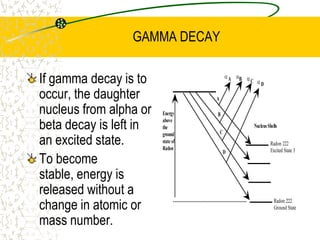
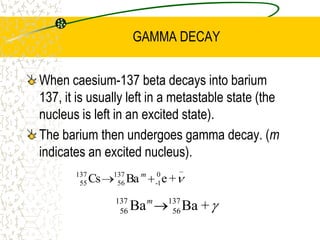
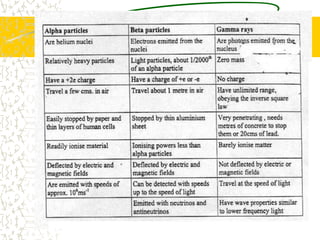
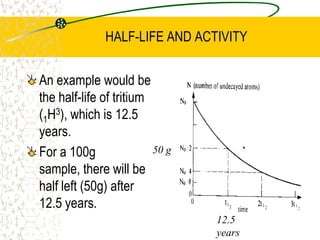
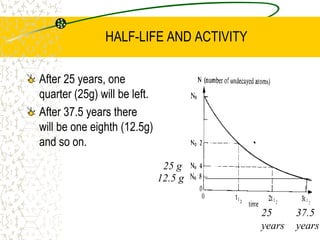
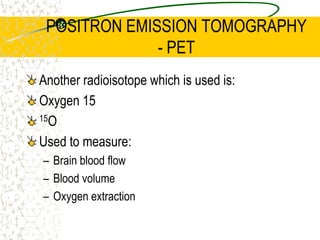
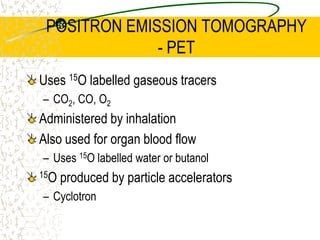
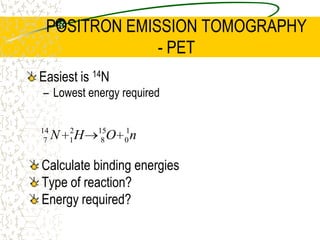
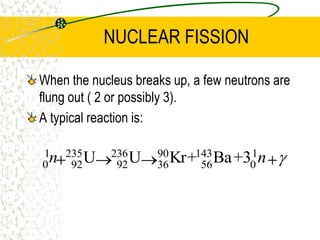
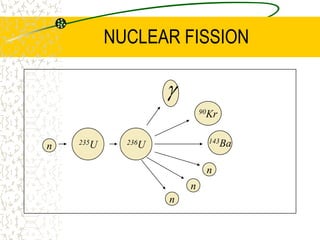
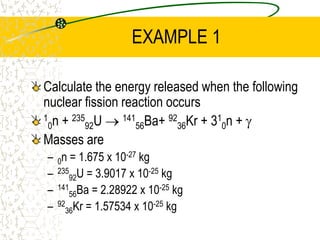
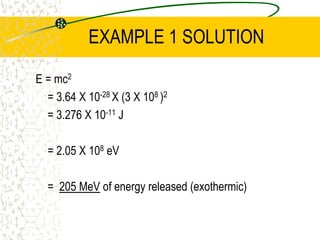
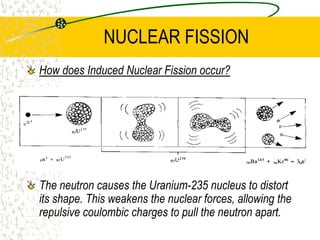
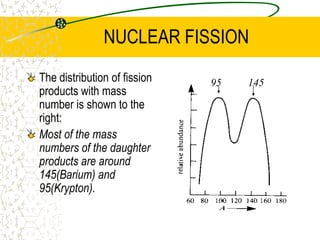
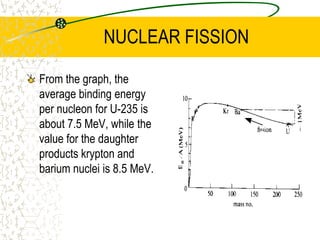
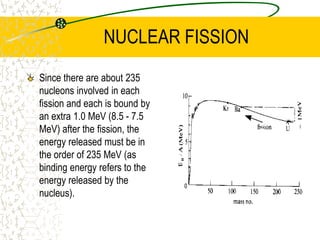
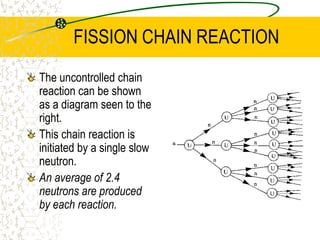
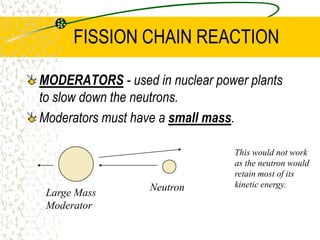
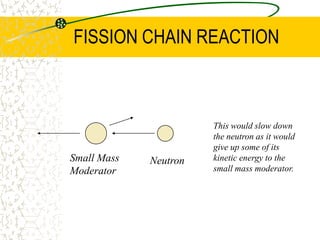
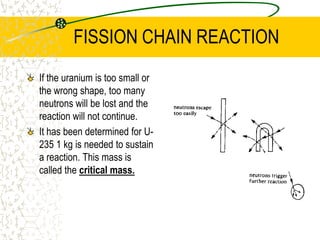
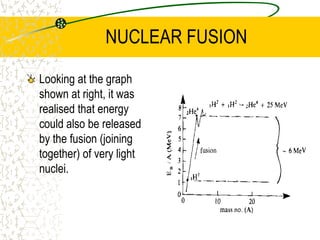
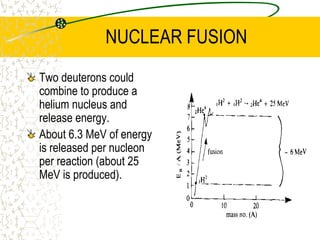
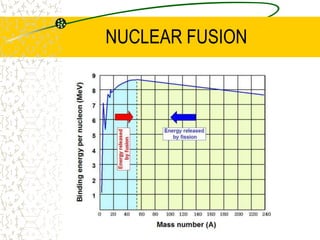
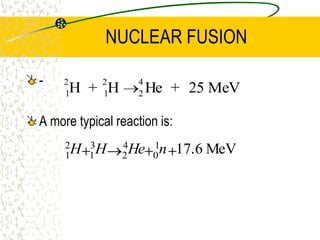
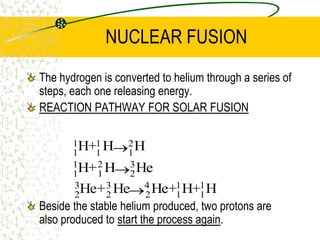
The document discusses line emission spectra, which are produced when electrons in excited gases fall to lower energy levels, emitting photons of specific frequencies. A key example is the spectral lines produced by burning sodium. Line emission spectra are unique for each element and can be used to identify unknown substances. The Bohr model of the atom postulated that electron energy is quantized, explaining the existence of line spectra.