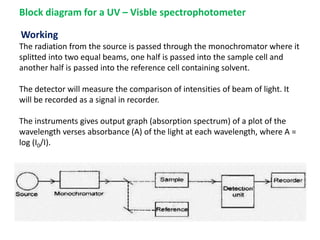
This document provides an introduction to ultraviolet spectroscopy. It discusses various topics including:
- The basic principles of spectroscopy and how it involves the interaction of electromagnetic radiation with matter.
- The two main types of spectroscopy: atomic spectroscopy which deals with atoms and molecular spectroscopy which deals with molecules.
- The two main classes of spectra - absorption spectra which measures decrease in radiation intensity, and emission spectra from excited state emission.
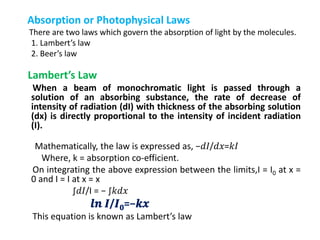
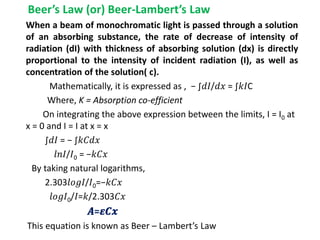
- Important absorption laws including Lambert's Law, Beer's Law, and how they relate intensity decrease to concentration and path length.
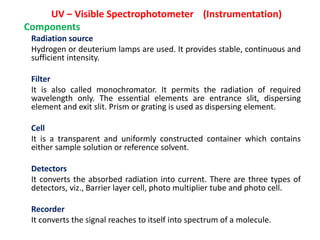
- How UV-Visible spectroscopy involves electronic transitions in molecules detected in the UV-Visible range.
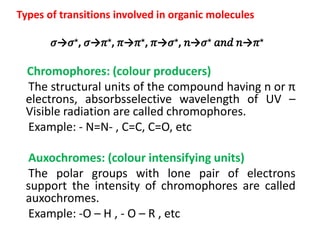
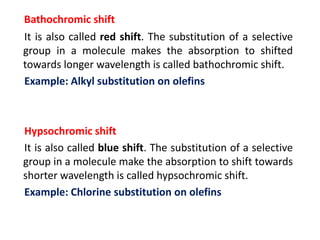
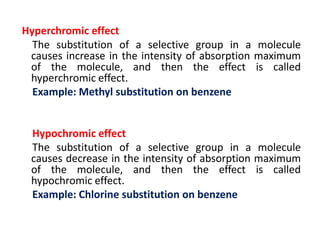
- Key concepts like chromophores, aux