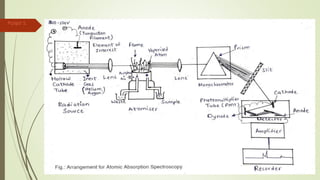
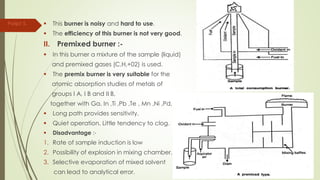
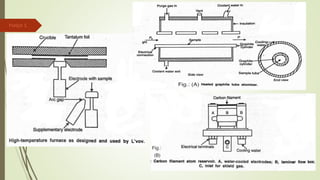
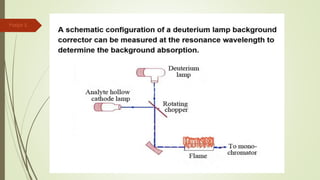
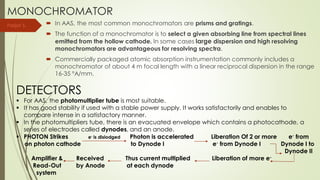
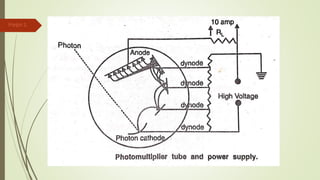
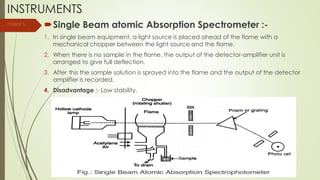
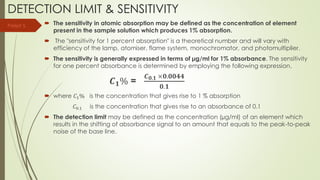
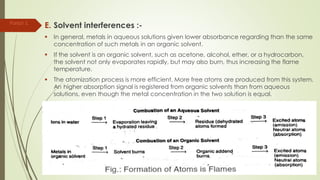
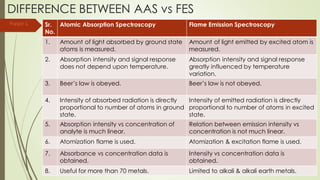
Atomic absorption spectroscopy is a technique used to determine the concentration of metal elements in liquids. It works by vaporizing the metal elements in a flame and measuring how much light of a specific wavelength is absorbed. The amount of absorption is directly proportional to the concentration of the metal. Key aspects of atomic absorption spectroscopy include using a hollow cathode lamp or electrodeless discharge lamp as a radiation source, a flame or carbon atomizer to vaporize the sample, and a photomultiplier detector to measure light absorption. It is a sensitive technique that can detect metals down to the parts-per-million level.