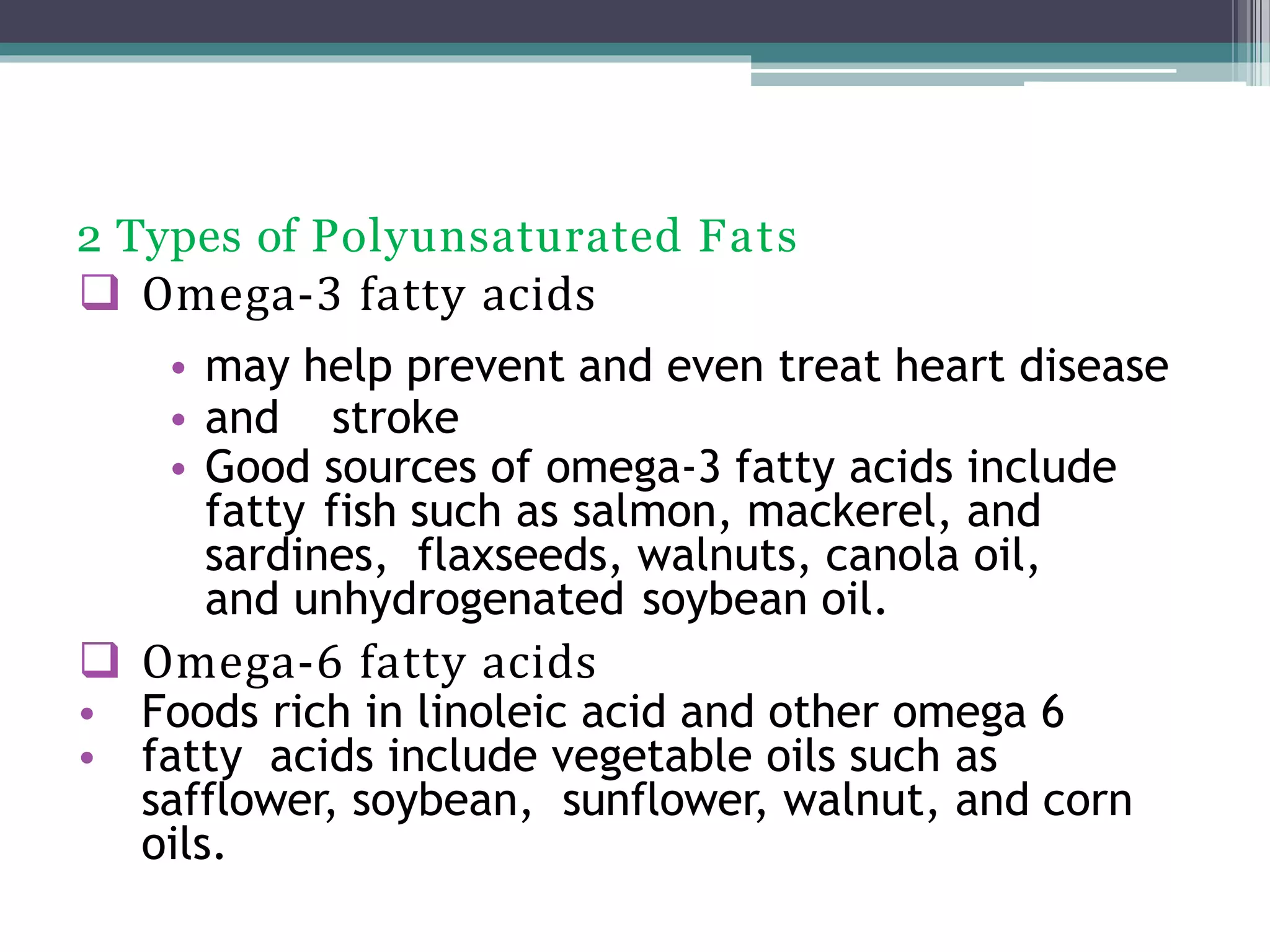
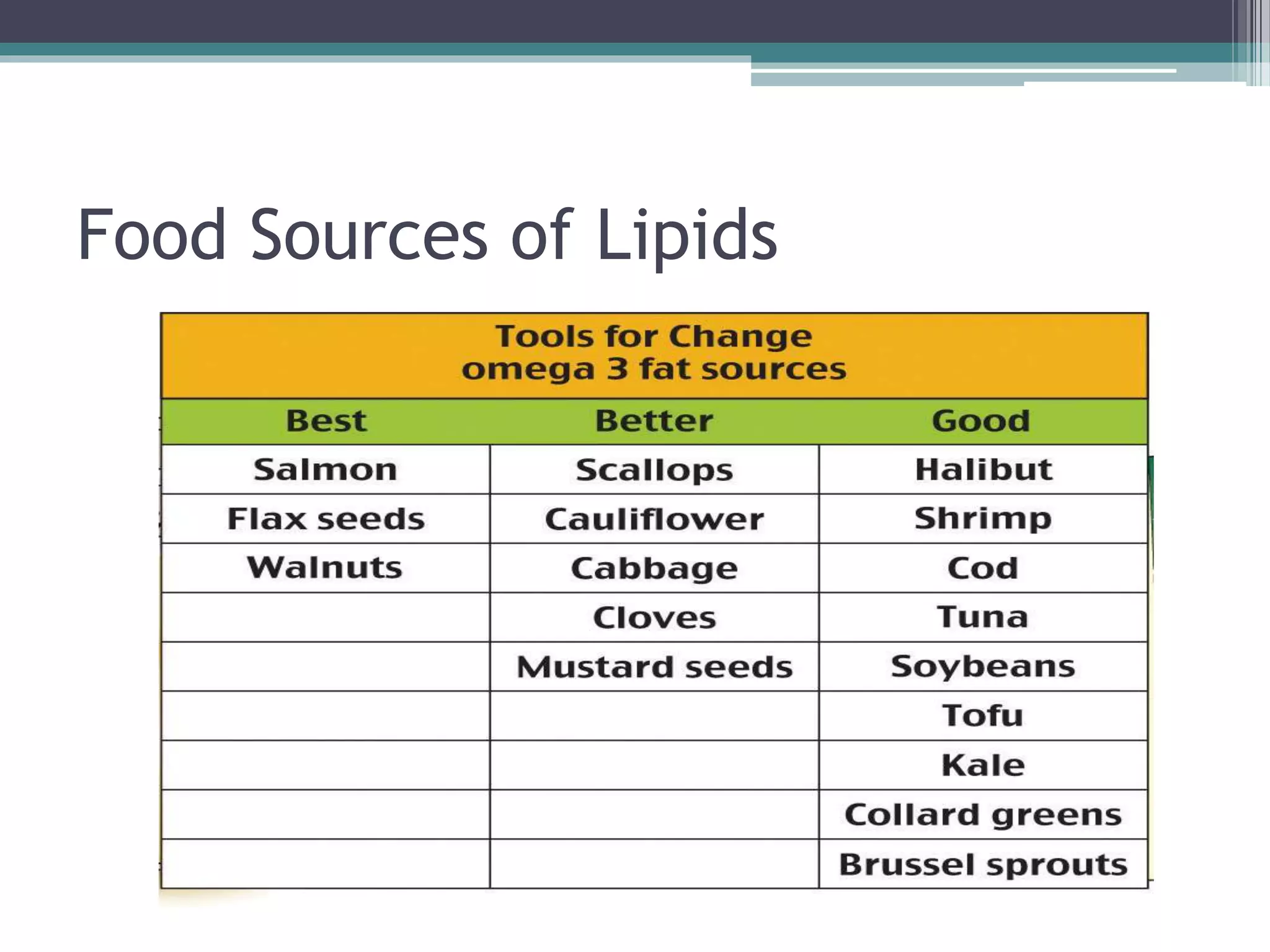
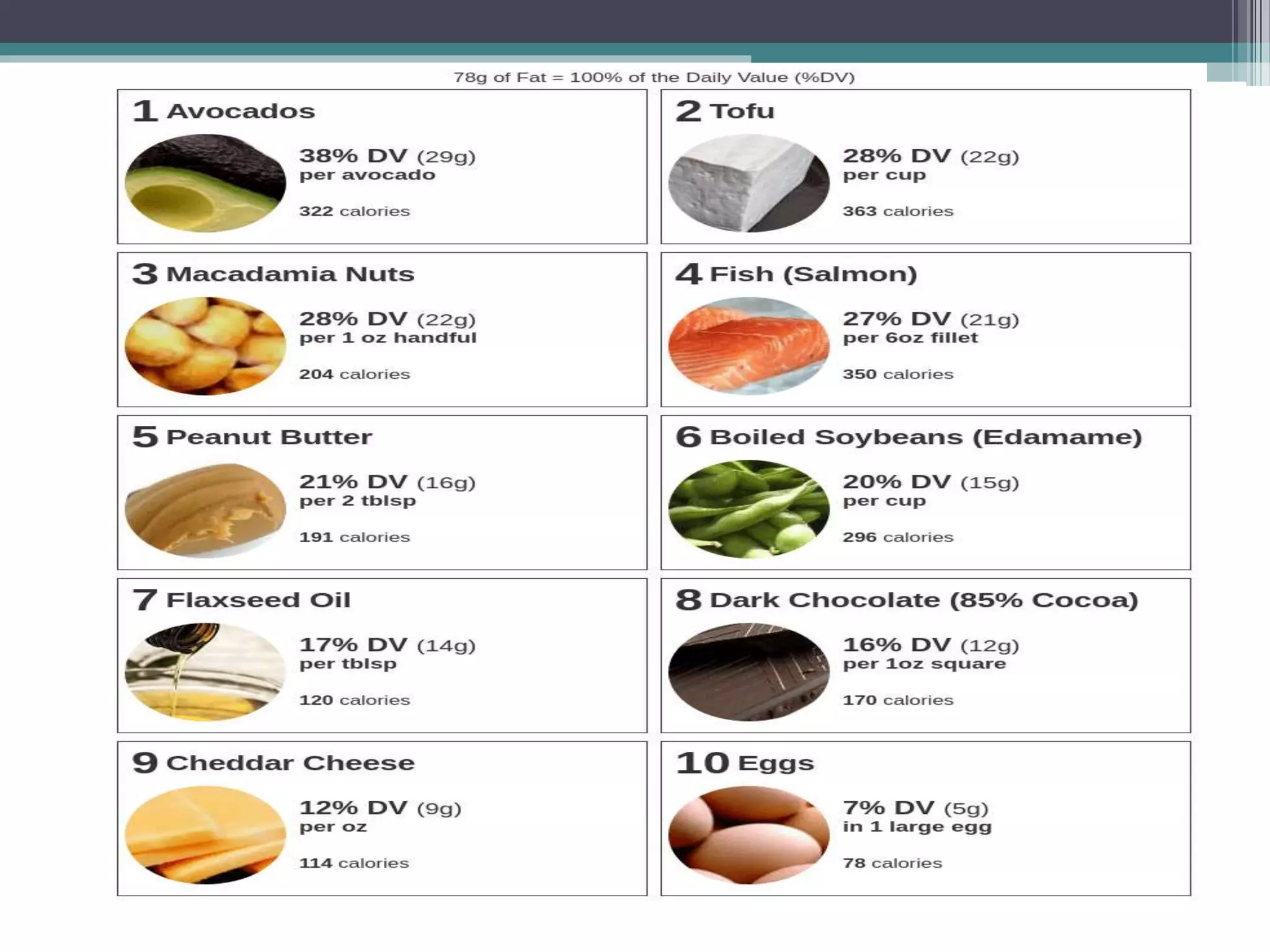
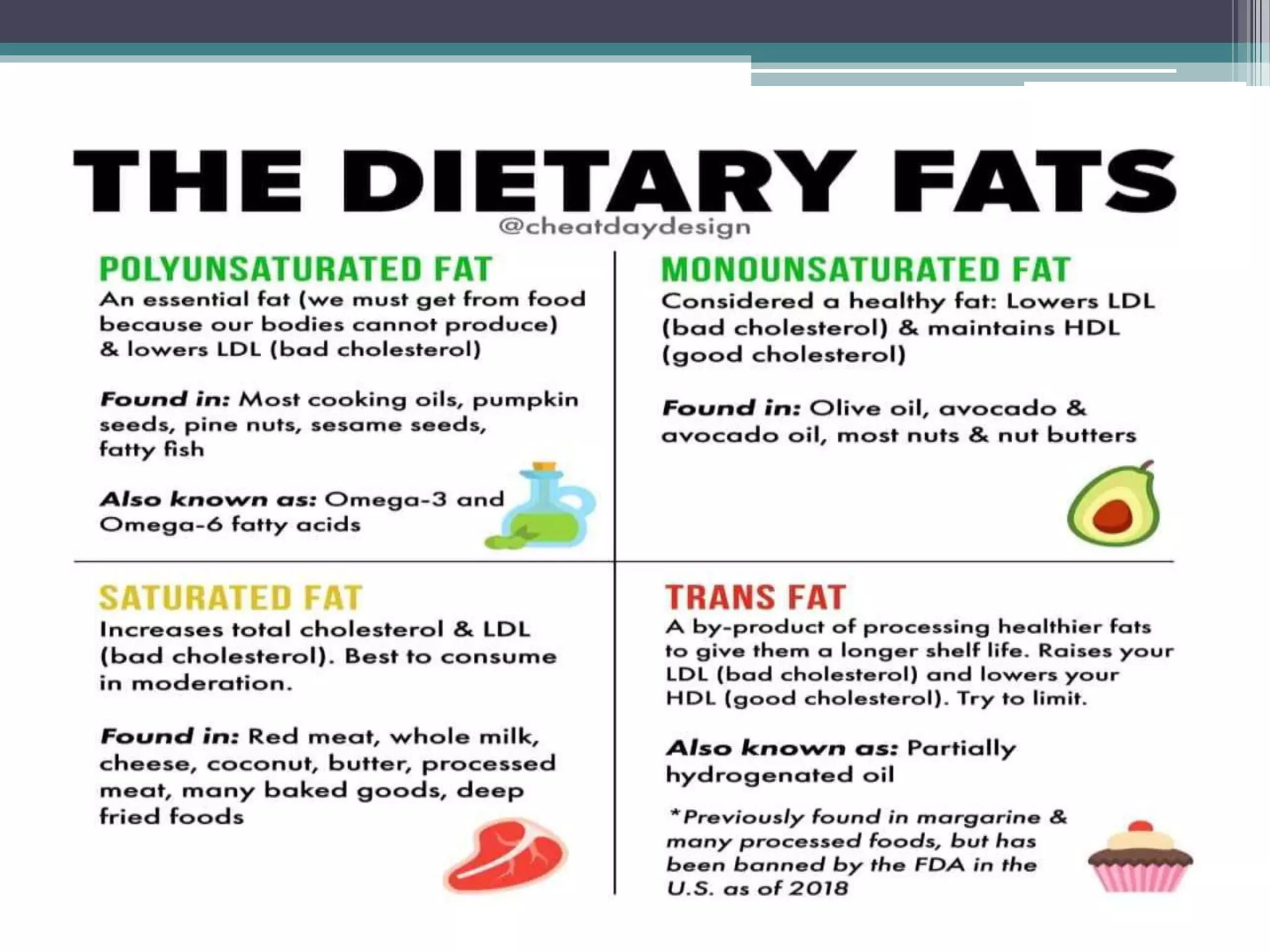
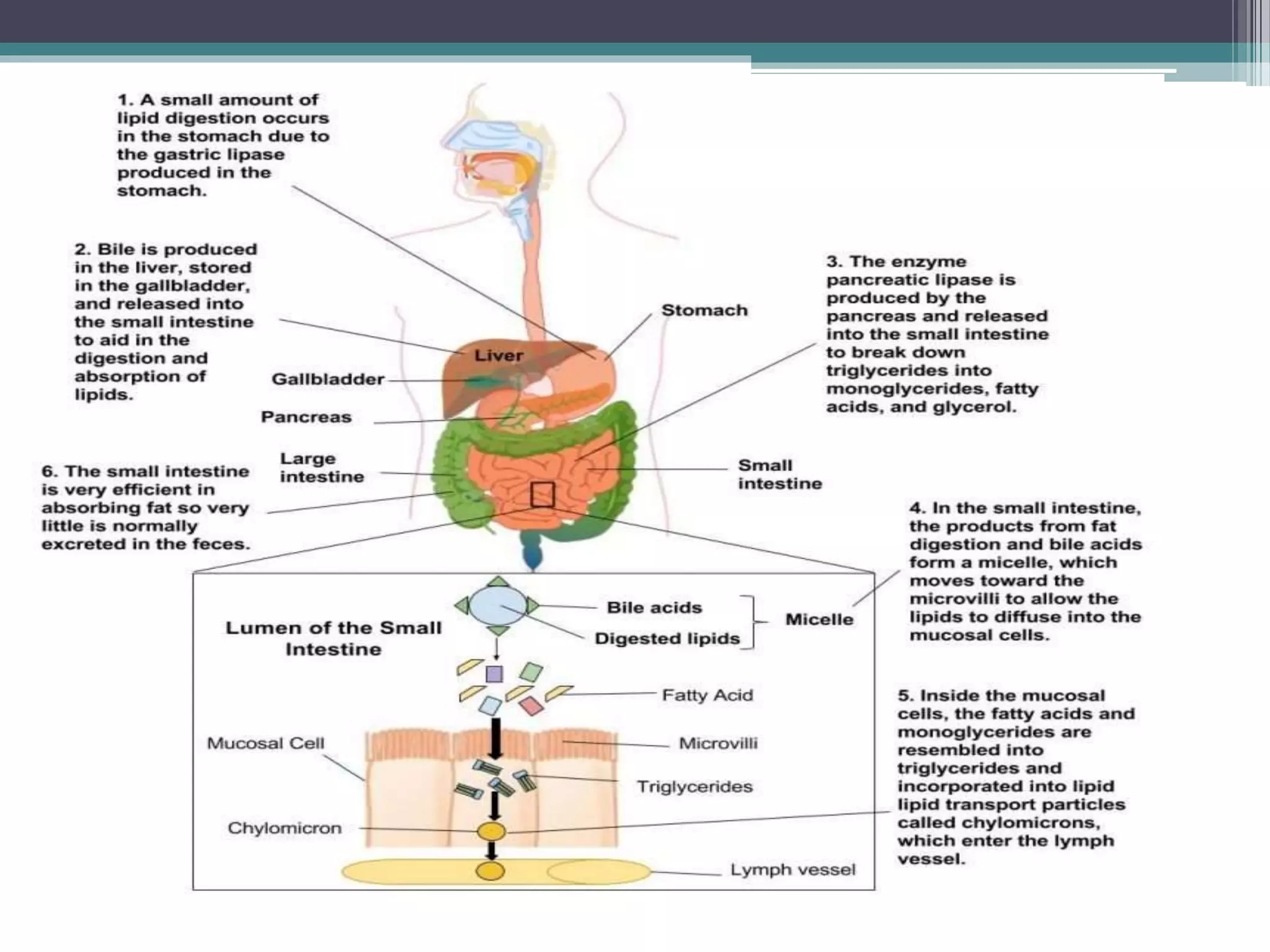
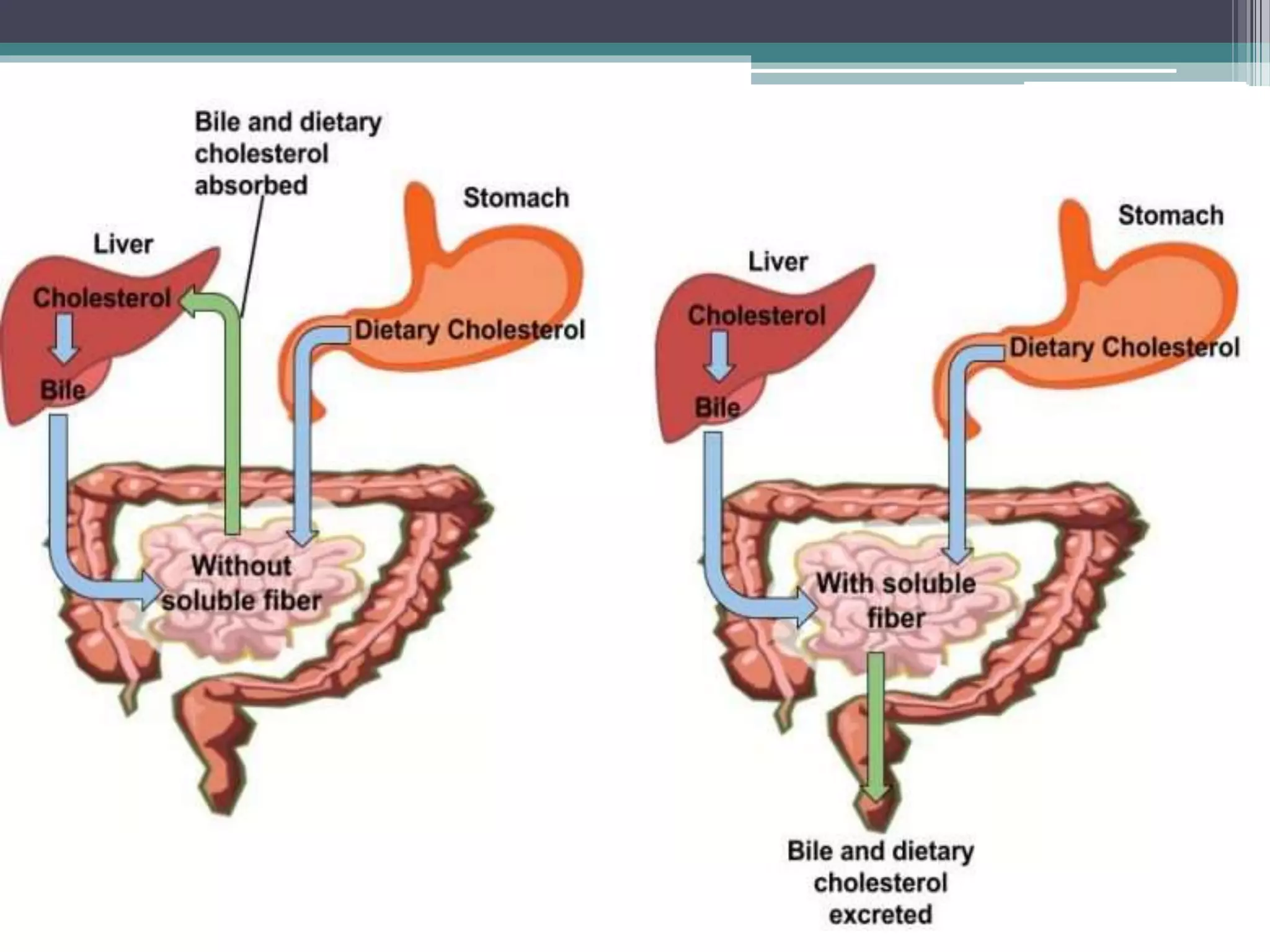
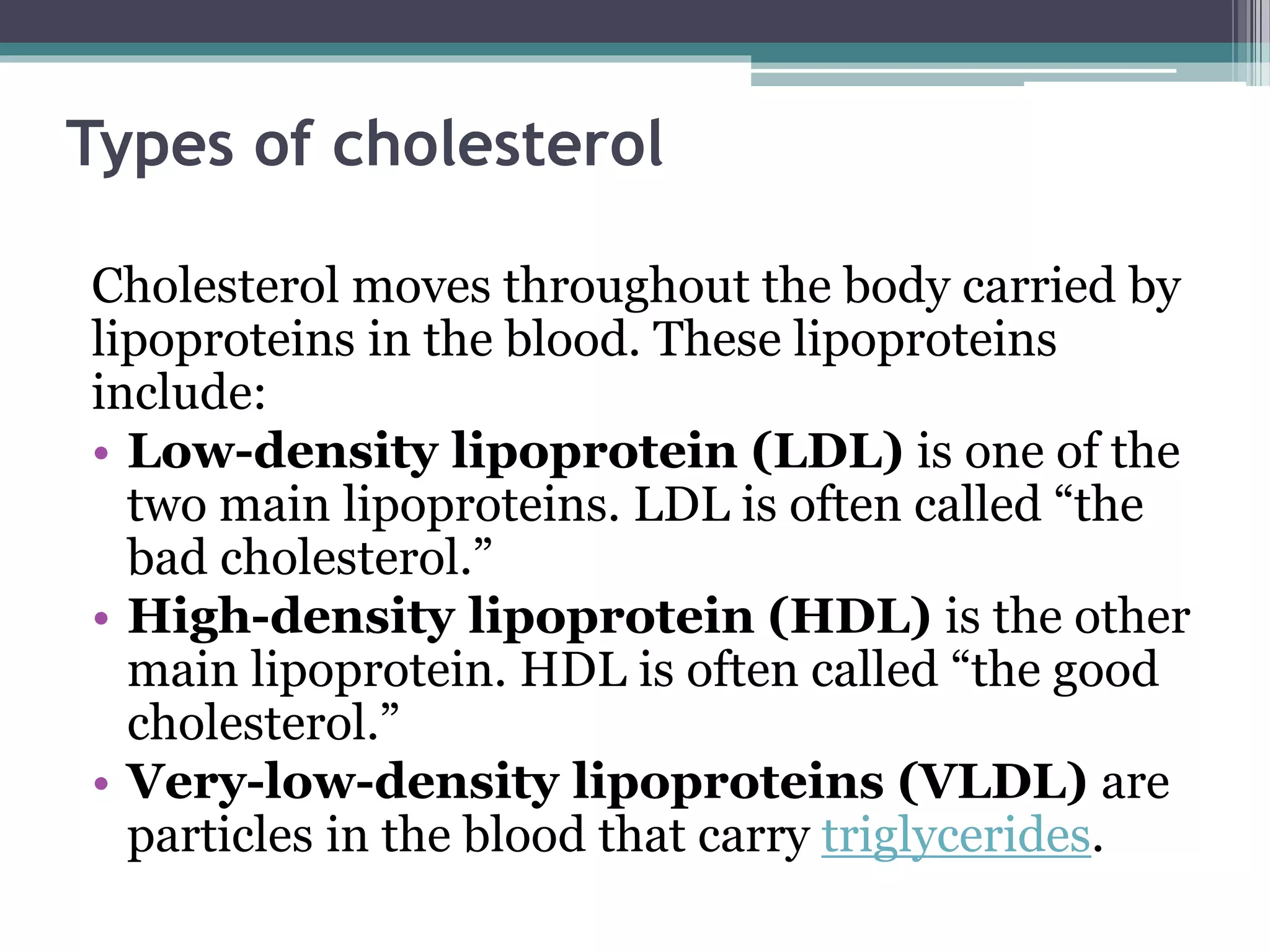
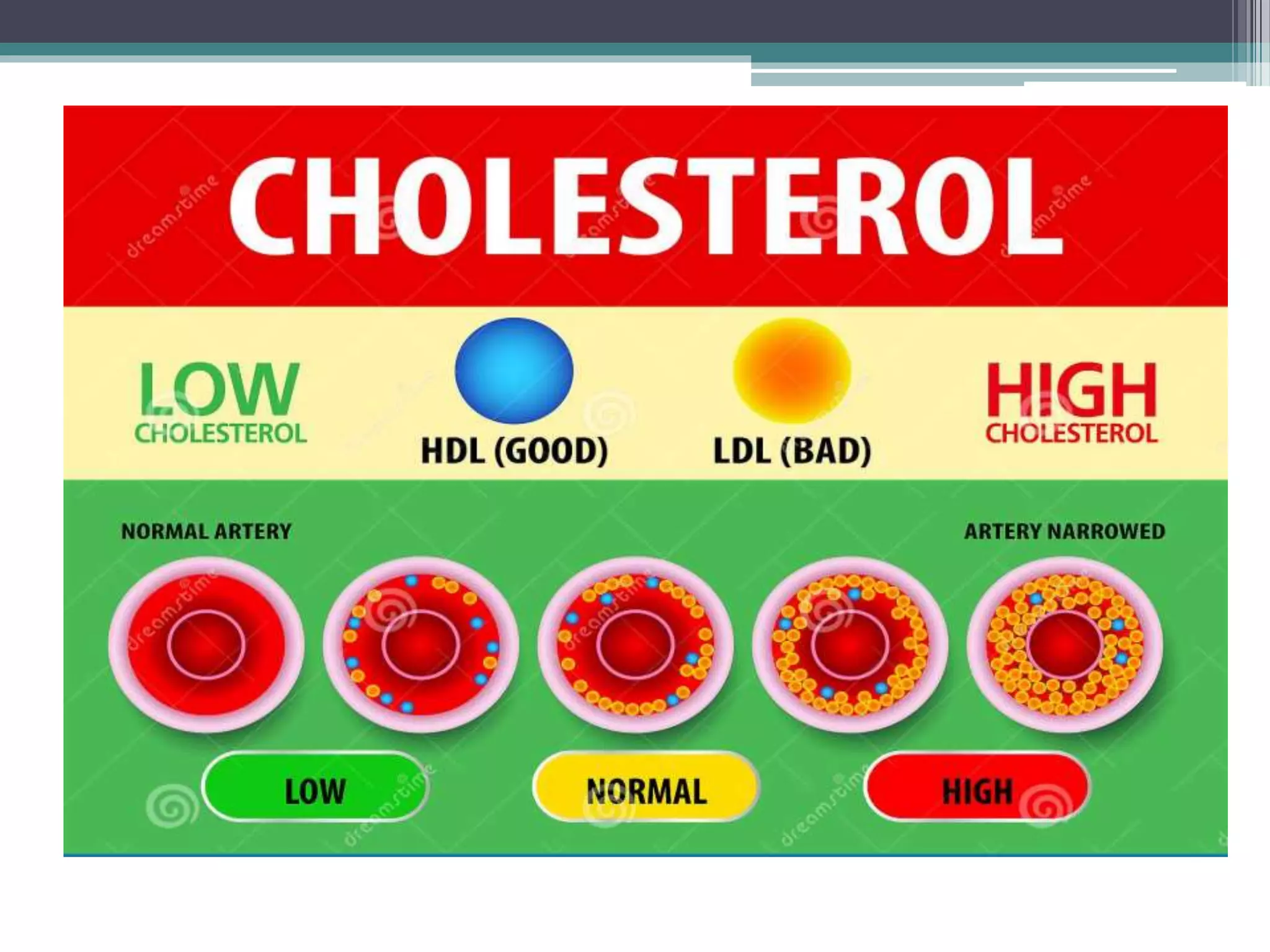
This document provides information about lipids and fats. It begins by defining lipids and explaining that they are a major building block of animal cells. It then discusses the different types of lipids, including simple lipids like fats and oils, complex lipids like phospholipids, and derived lipids like cholesterol. The document explains the classification and functions of various lipids such as phospholipids, essential fatty acids, and saturated and unsaturated fatty acids. It also covers the digestion and absorption of lipids, as well as the different types of cholesterol and their importance.