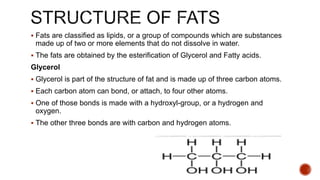
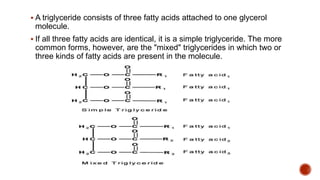
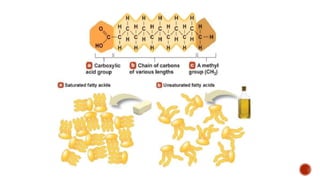
Fats are large molecules classified as lipids, crucial for energy storage, vitamin absorption, and various functions in food. They can be saturated or unsaturated and consist of glycerol and fatty acids, primarily forming triglycerides. Fats play an essential role in health and nutrition, influencing food texture and flavor, and are extracted through rendering, pressing, or solvent extraction methods.