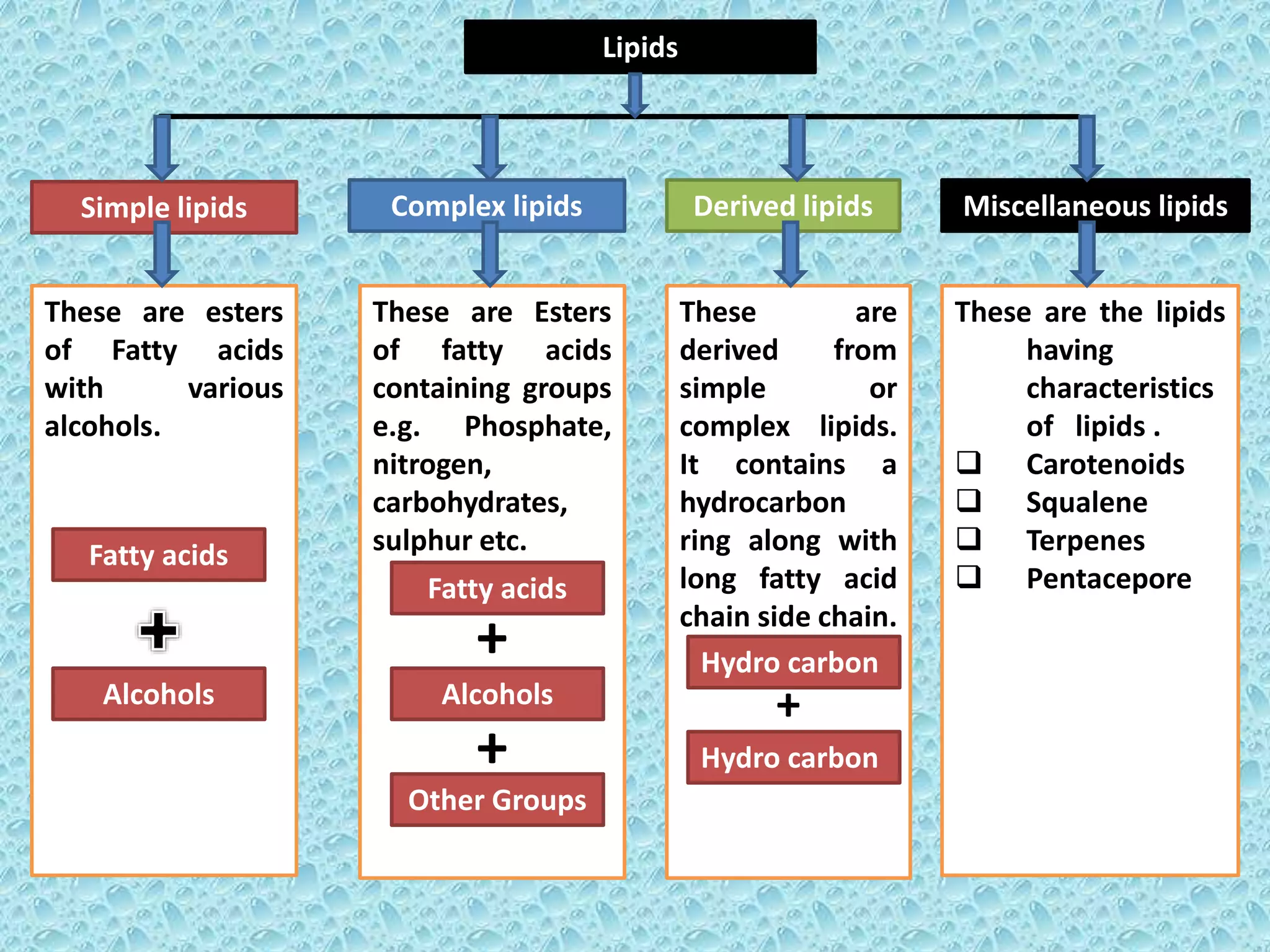
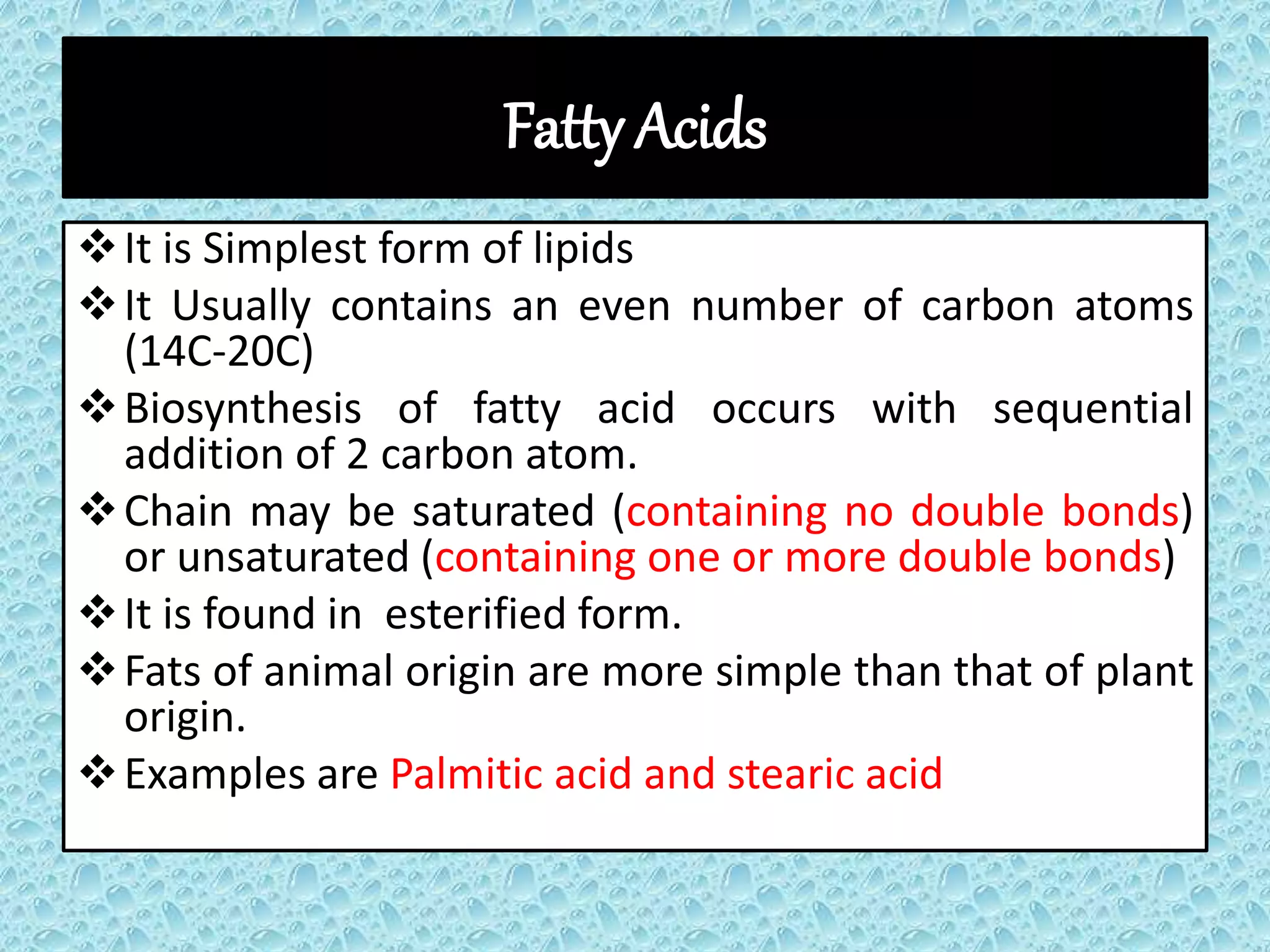
Lipids are macrobiomolecules derived from the Greek word 'lipos', playing crucial roles in energy storage, signaling, and structural components of cell membranes. They are categorized into four types: simple, compound, derived, and miscellaneous, each with specific subtypes such as fatty acids, phospholipids, and steroids. Understanding lipids is essential due to their multifaceted applications in biochemistry, nutrition, and industry.