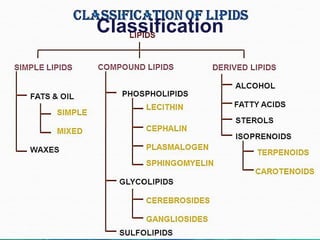
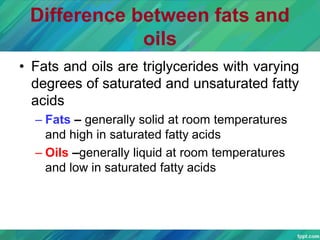
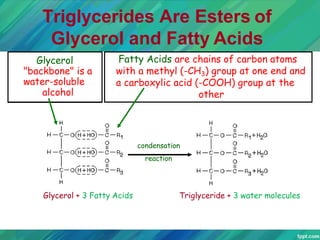
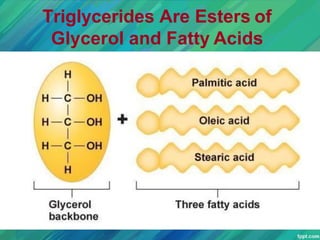
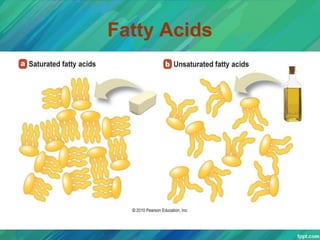
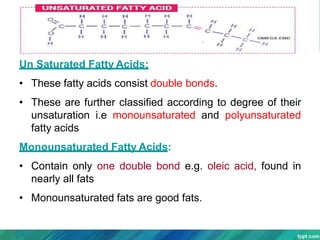
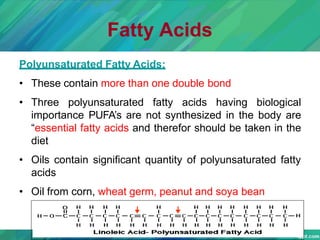
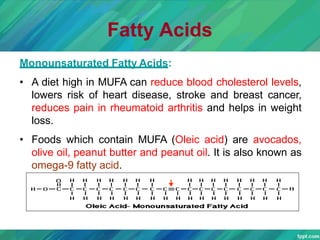
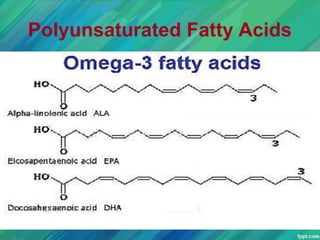
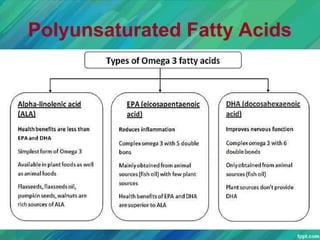
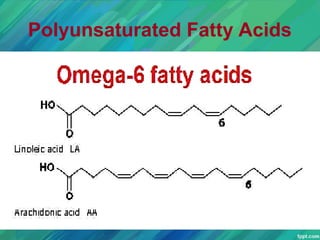
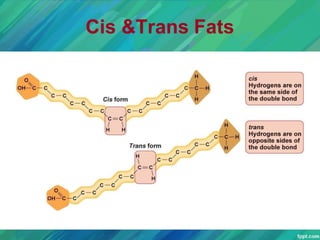
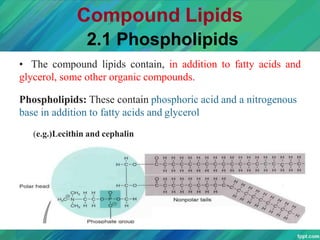
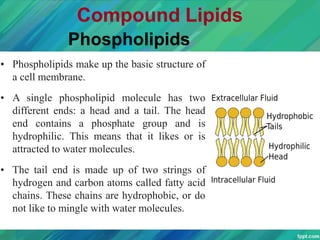
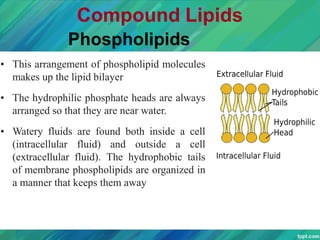
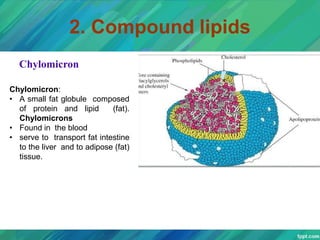
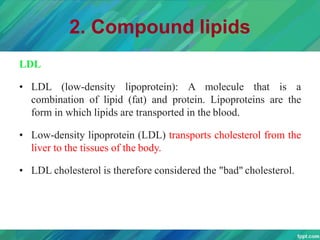
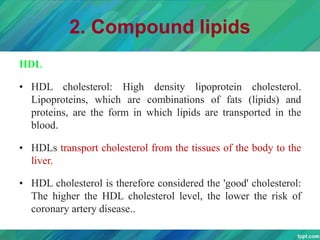
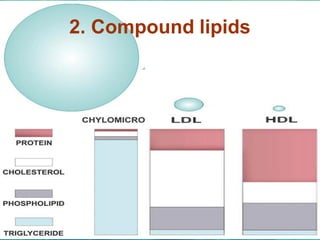
Lipids are organic compounds that are insoluble in water but soluble in organic solvents. They include fats, oils, waxes, sterols and phospholipids. Fats and oils are triglycerides composed of glycerol and fatty acids. Fatty acids are classified as saturated, monounsaturated, or polyunsaturated. Phospholipids are a major component of cell membranes and lipoproteins transport lipids in the blood. Lipids serve important functions as energy stores, insulation, and as precursors to other compounds like hormones and vitamins.